
Bundle: Chemistry, 10th + Laboratory Handbook for General Chemistry, 3rd + Student Resource Center Printed Access Card + Student Solutions Manual for ... Access Card for Zumdahl/Zumdahl/DeCoste
10th Edition
ISBN: 9781337816465
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 83E
The decomposition of many substances on the surface of a heterogeneous catalyst shows the following behavior:
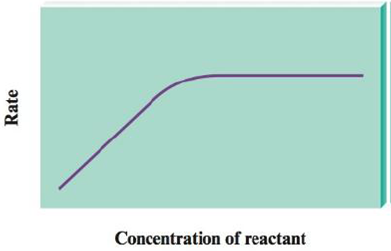
How do you account for the rate law changing from first order to zero order in the concentration of reactant?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
9
alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo-
O States of Matter
Sketching a described thermodynamic change on a phase diagram
0/5
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
pressure (atm)
3-
200
temperature (K)
Explanation
Chick
Q Sownch
0+
aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm
O States of Matter
Sketching a described thermodynamic change on a phase diagram
0/5
Gab
The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the
pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes.
F3
pressure (atm)
0-
0
200
Explanation
temperature (K)
Check
F4
F5
☀+
Q Search
Chill Will an
9
ENG
F6
F7
F8
F9
8
Delete
F10
F11
F12
Insert
PrtSc
114
d
Ar
Chapter 12 Solutions
Bundle: Chemistry, 10th + Laboratory Handbook for General Chemistry, 3rd + Student Resource Center Printed Access Card + Student Solutions Manual for ... Access Card for Zumdahl/Zumdahl/DeCoste
Ch. 12 - Define reaction rate. Distinguish between the...Ch. 12 - Distinguish between the differential rate law and...Ch. 12 - One experimental procedure that can be used to...Ch. 12 - The initial rate for a reaction is equal to the...Ch. 12 - Consider the zero-, first-, and second-order...Ch. 12 - Derive expressions for the half-life of zero-,...Ch. 12 - Prob. 7RQCh. 12 - What two requirements must be met to call a...Ch. 12 - Prob. 9RQCh. 12 - Give the Arrhenius equation. Take the natural log...
Ch. 12 - Why does a catalyst increase the rate of a...Ch. 12 - Define stability from both a kinetic and...Ch. 12 - Describe at least two experiments you could...Ch. 12 - Make a graph of [A] versus time for zero-, first-,...Ch. 12 - How does temperature affect k, the rate constant?...Ch. 12 - Consider the following statements: In general, the...Ch. 12 - For the reaction A+BC, explain at least two ways...Ch. 12 - A friend of yours states, A balanced equation...Ch. 12 - Provide a conceptual rationale for the differences...Ch. 12 - The rate constant (k) depends on which of the...Ch. 12 - Table 11-2 illustrates how the average rate of a...Ch. 12 - The rate law for a reaction can be determined only...Ch. 12 - The plot below shows the number of collisions with...Ch. 12 - For the reaction O2(g)+2NO(g)2NO2(g) the observed...Ch. 12 - Each of the statements given below is false....Ch. 12 - Define what is meant by unimolecular and...Ch. 12 - The type of rate law for a reaction, either the...Ch. 12 - The initial rate of a reaction doubles as the...Ch. 12 - Hydrogen reacts explosively with oxygen. However,...Ch. 12 - The central idea of the collision model is that...Ch. 12 - Consider the following energy plots for a chemical...Ch. 12 - Prob. 21QCh. 12 - Prob. 22QCh. 12 - The combustion of carbohydrates and the combustion...Ch. 12 - Would the slope of a ln(k) versus 1/T plot (with...Ch. 12 - Consider the reaction 4PH3(g)P4(g)+6H2(g) If, in a...Ch. 12 - In the Haber process for the production of...Ch. 12 - At 40C, H2O2 (aq) will decompose according to the...Ch. 12 - Consider the general reaction aA+bBcC and the...Ch. 12 - What are the units for each of the following if...Ch. 12 - The rate law for the reaction...Ch. 12 - The reaction 2NO(g)+Cl2(g)2NOCl(g) was studied at...Ch. 12 - The reaction 2I-(aq)+S2O82-(aq)I2(aq)+2SO42-(aq)...Ch. 12 - The decomposition of nitrosyl chloride was...Ch. 12 - The following data were obtained for the gas-phase...Ch. 12 - The reaction I(aq)+OCl(aq)IO(aq)+Cl(aq) was...Ch. 12 - The reaction 2NO(g)+O2(g)2NO2(g) was studied. and...Ch. 12 - The rote of the reaction between hemoglobin (Hb)...Ch. 12 - The following data were obtained for the reaction...Ch. 12 - The decomposition of hydrogen peroxide was...Ch. 12 - A certain reaction has the following general form:...Ch. 12 - The rate of the reaction NO2(g)+CO(g)NO(g)+CO2(g)...Ch. 12 - A certain reaction has the following general form:...Ch. 12 - The decomposition of ethanol (C2H5OH) on an...Ch. 12 - At 500 K in the presence of a copper surface,...Ch. 12 - The dimerization of butadiene 2C4H6(g)C8H12(g) was...Ch. 12 - The rate of the reaction O(g)+NO2(g)NO(g)+O2(g)...Ch. 12 - Experimental data for the reaction A2B+C have been...Ch. 12 - Prob. 48ECh. 12 - The reaction AB+C is known to be zero order in A...Ch. 12 - The decomposition of hydrogen iodide on finely...Ch. 12 - Prob. 51ECh. 12 - A first-order reaction is 75.0% complete in 320....Ch. 12 - The rate law for the decomposition of phosphine...Ch. 12 - DDT (molar mass = 354.49 g/mol) was a widely used...Ch. 12 - The rate law for the reaction...Ch. 12 - Prob. 57ECh. 12 - Theophylline is a pharmaceutical drug that is...Ch. 12 - You and a coworker have developed a molecule...Ch. 12 - Consider the hypothetical reaction A+B+2C2D+3E...Ch. 12 - Write the rate laws for the following elementary...Ch. 12 - A possible mechanism for the decomposition of...Ch. 12 - A proposed mechanism for a reaction is...Ch. 12 - The mechanism for the gas-phase reaction of...Ch. 12 - Is the mechanism NO+Cl2l1NOCl2NOCl2+NOl22NOCl...Ch. 12 - The reaction 2NO(g) + O2(g) 2NO2(g) exhibits the...Ch. 12 - For the following reaction profile, indicate a....Ch. 12 - Draw a rough sketch of the energy profile for each...Ch. 12 - The activation energy for the reaction...Ch. 12 - The activation energy for some reaction...Ch. 12 - The rate constant for the gas-phase decomposition...Ch. 12 - The reaction (CH3)3CBr+OH(CH3)3COH+Br in a certain...Ch. 12 - The activation energy for the decomposition of...Ch. 12 - A first-order reaction has rate constants of 4.6 ...Ch. 12 - A certain reaction has an activation energy of...Ch. 12 - Prob. 76ECh. 12 - Which of the following reactions would you expect...Ch. 12 - Prob. 78ECh. 12 - One mechanism for the destruction of ozone in the...Ch. 12 - One of the concerns about the use of Freons is...Ch. 12 - Assuming that the mechanism for the hydrogenation...Ch. 12 - The decomposition of NH3 to N2 and H2 was studied...Ch. 12 - The decomposition of many substances on the...Ch. 12 - Prob. 84ECh. 12 - A popular chemical demonstration is the magic...Ch. 12 - Prob. 86ECh. 12 - Consider the following representation of the...Ch. 12 - The reaction H2SeO3(aq) + 6I-(aq) + 4H+(aq) Se(s)...Ch. 12 - Consider two reaction vessels, one containing A...Ch. 12 - Sulfuryl chloride (SO2Cl2) decomposes to sulfur...Ch. 12 - For the reaction 2N2O5(g)4NO2(g)+O2(g) the...Ch. 12 - Experimental values for the temperature dependence...Ch. 12 - At 620. K butadiene dimerizes at a moderate rate....Ch. 12 - For a first order gas phase reaction A products,...Ch. 12 - Cobra venom helps the snake secure food by binding...Ch. 12 - Iodomethane (CH3I) is a commonly used reagent in...Ch. 12 - Experiments during a recent summer on a number of...Ch. 12 - The activation energy of a certain uncatalyzed...Ch. 12 - Consider the following initial rate data for the...Ch. 12 - Consider a hypothetical reaction between A and B:...Ch. 12 - Consider the reaction 3A+B+CD+E where the rate law...Ch. 12 - The thiosulfate ion (S2O32) is oxidized by iodine...Ch. 12 - The reaction A(aq)+B(aq)products(aq) was studied,...Ch. 12 - A certain substance, initially present at 0.0800...Ch. 12 - A reaction of the form aAProducts gives a plot of...Ch. 12 - A certain reaction has the form aAProducts At a...Ch. 12 - Which of the following statement(s) is( are) true?...Ch. 12 - Consider the hypothetical reaction A2(g) + B2(g) ...Ch. 12 - Experiments have shown that the average frequency...Ch. 12 - Consider a reaction of the type aA products, in...Ch. 12 - A study was made of the effect of the hydroxide...Ch. 12 - Two isomers (A and B) of a given compound dimerize...Ch. 12 - The reaction NO(g)+O3NO2(g)+O2(g) was studied by...Ch. 12 - Prob. 114CPCh. 12 - In the gas phase, the production of phosgene from...Ch. 12 - Most reactions occur by a series of steps. The...Ch. 12 - You are studying the kinetics of the reaction...Ch. 12 - The decomposition of NO2(g) occurs by the...Ch. 12 - The following data were collected in two studies...Ch. 12 - Consider the following hypothetical data collected...Ch. 12 - Consider the hypothetical reaction A+B+2C2D+3E In...Ch. 12 - Hydrogen peroxide and the iodide ion react in...Ch. 12 - Sulfuryl chloride undergoes first-order...Ch. 12 - Upon dissolving InCl(s) in HCl, In+(aq) undergoes...Ch. 12 - The decomposition of iodoethane in the gas phase...Ch. 12 - Consider the following reaction: CH3X+YCH3Y+X At...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does trandlation differ from transcription?
Microbiology: Principles and Explorations
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- x + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- Results Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forward
- Consider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forwardStarting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward
- - The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forwardDraw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning  Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY