
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
8th Edition
ISBN: 9780100547964
Author: Hurley
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 74QAP
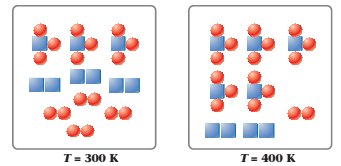
The figures below represent the following reaction at equilibrium at different temperatures.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Polar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solvents
Deducing the Peactants
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one
step, by moderately heating the reactants?
?
Δ
If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any
arrangement you like.
If your answer is no, check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a structure.
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
X
Draw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)
Chapter 12 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
Ch. 12 - The following data are for the system A(g)2B(g)...Ch. 12 - The following data are for the system A(g)2B(g)...Ch. 12 - Prob. 3QAPCh. 12 - Complete the table below for the reaction...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Given the following descriptions of reversible...Ch. 12 - Given the following descriptions of reversible...
Ch. 12 - Write an equation for an equilibrium system that...Ch. 12 - Write a chemical equation for an equilibrium...Ch. 12 - Consider the following reaction at 250C:...Ch. 12 - Consider the following reaction at 1000 C:...Ch. 12 - At 627C, K=0.76 for the reaction...Ch. 12 - At 800C, K=2.2104 for the following reaction...Ch. 12 - Prob. 17QAPCh. 12 - Given the following data at 25C...Ch. 12 - Given the following data at a certain temperature,...Ch. 12 - Consider the following hypothetical reactions and...Ch. 12 - When one mole of carbon disulfide gas reacts with...Ch. 12 - Calculate K for the formation of methyl alcohol at...Ch. 12 - Ammonium carbamate solid (NH4CO2NH2) decomposes at...Ch. 12 - Consider the decomposition at 25C of one mole of...Ch. 12 - Consider the decomposition of ammonium hydrogen...Ch. 12 - A sealed flask has 0.541 atm of SO3 at 1000 K. The...Ch. 12 - A gaseous reaction mixture contains 0.30 atm SO2,...Ch. 12 - For the system PCl5(g)PCl3(g)+Cl2(g)K is 26 at...Ch. 12 - The reversible reaction between hydrogen chloride...Ch. 12 - The reversible reaction between hydrogen chloride...Ch. 12 - A compound, X, decomposes at 131C according to the...Ch. 12 - Consider the following reaction at 75C:...Ch. 12 - Consider the reaction between nitrogen and steam:...Ch. 12 - At 500C, k for the for the formation of ammonia...Ch. 12 - At a certain temperature, K is 4.9 for the...Ch. 12 - At a certain temperature, K=0.29 for the...Ch. 12 - For the reaction N2(g)+2H2O(g)2NO(g)+2H2(g) K is...Ch. 12 - Nitrogen dioxide can decompose to nitrogen oxide...Ch. 12 - Consider the following reaction:...Ch. 12 - Consider the hypothetical reaction at 325C...Ch. 12 - At a certain temperature, the equilibrium constant...Ch. 12 - At 460C, the reaction SO2(g)+NO2(g)NO(g)+SO3(g)...Ch. 12 - Solid ammonium iodide decomposes to ammonia and...Ch. 12 - Consider the following decomposition at 80C....Ch. 12 - Hydrogen cyanide, a highly toxic gas, can...Ch. 12 - At 800 K, hydrogen iodide can decompose into...Ch. 12 - For the following reactions, predict whether the...Ch. 12 - Follow the directions of Question 47 for the...Ch. 12 - Consider the system SO3(g)SO2(g)+12 O2(g)H=98.9kJ...Ch. 12 - Consider the system...Ch. 12 - Predict the direction in which each of the...Ch. 12 - Predict the direction in which each of the...Ch. 12 - At a certain temperature, nitrogen and oxygen...Ch. 12 - Consider the following hypothetical reaction:...Ch. 12 - Iodine chloride decomposes at high temperatures to...Ch. 12 - Sulfur oxychloride, SO2Cl2, decomposes to sulfur...Ch. 12 - For the following reaction C(s)+2H2(g)CH4(g)...Ch. 12 - For the system 2SO3(g)2SO2(g)+O2(g) K=1.32 at 627....Ch. 12 - For a certain reaction, H is +33 kJ. What is the...Ch. 12 - Prob. 60QAPCh. 12 - Hemoglobin (Hb) binds to both oxygen and carbon...Ch. 12 - Mustard gas, used in chemical warfare in World War...Ch. 12 - Prob. 63QAPCh. 12 - For the decomposition of CaCO3 at 900C, K=1.04....Ch. 12 - Isopropyl alcohol is the main ingredient in...Ch. 12 - Consider the equilibrium H2(g)+S(s)H2S(g)When this...Ch. 12 - Prob. 67QAPCh. 12 - The following data apply to the unbalanced...Ch. 12 - Consider the reaction: A(g)+2B(g)+C(s)2D(g)At 25C,...Ch. 12 - For the reaction C(s)+CO2(g)2CO(g) K=168 at 1273...Ch. 12 - Consider the system A(g)+2B(g)+C(g)2D(g)at 25C. At...Ch. 12 - The graph below is similar to that of Figure 12.2....Ch. 12 - Prob. 73QAPCh. 12 - The figures below represent the following reaction...Ch. 12 - Prob. 75QAPCh. 12 - Prob. 76QAPCh. 12 - Consider the following reaction at a certain...Ch. 12 - Prob. 78QAPCh. 12 - Ammonia can decompose into its constituent...Ch. 12 - Hydrogen iodide gas decomposes to hydrogen gas and...Ch. 12 - For the system SO3(g)SO2(g)+12 O2(g)at 1000 K,...Ch. 12 - A student studies the equilibrium I2(g)2I(g)at a...Ch. 12 - At a certain temperature, the reaction...Ch. 12 - Benzaldehyde, a flavoring agent, is obtained by...Ch. 12 - Prob. 85QAPCh. 12 - Prob. 86QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Bookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forwardDeducing the reactants of a Diels-Alder reaction n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. >arrow_forwardPredict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forward
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardreciprocal lattices rotates along with the real space lattices of the crystal. true or false?arrow_forwardDeducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY