
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 41E
Electron dot structure and structural formula of
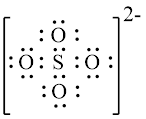
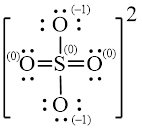
Explanation of Solution
In molecule,
Figure 1
Figure 2
Solid line, in Figure 2, between the atoms is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 41E
Electron dot structure and structural formula of
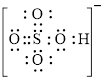
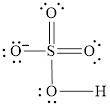
Explanation of Solution
In molecule
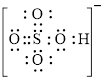
Figure 3

Figure 4
Each solid line, in Figure 4, between the atoms is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 41E
Electron dot structure and structural formula of
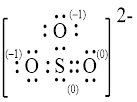
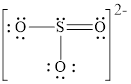
Explanation of Solution
In molecule
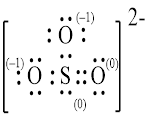
Figure 5

Figure 6
Each solid line, in Figure 6, between the atoms is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 41E
Electron dot structure and structural formula of
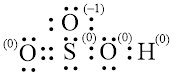
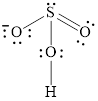
Explanation of Solution
In molecule
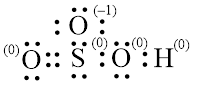
Figure 7

Figure 8
Each solid line, in Figure 8, between the atoms is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
EBK INTRODUCTORY CHEMISTRY
- 14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward:0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forward
- Draw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forward
- answer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forwardTrue or false, chemistryarrow_forward
- answer thse questions with mechanisms and steps. handwritten please!arrow_forwardC app.aktiv.com Draw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CH3Br Drawingarrow_forwardDraw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CHзBr Drawingarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

