
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
12th Edition
ISBN: 9781119304241
Author: Solomons
Publisher: WILEY C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 28P
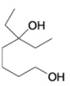
For each of the following alcohols, write a retrosunthetic analysis and synthesis that involves an appropriate organometallic reagent (either a Grignard or alkyllithium reagent).
(a)
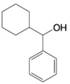
(b)
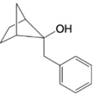
(c)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a)
NHBoc
⚫OBn
HO.
H3C
CO2CH3
-OBn
H3C
H3C.
H3C.
NHBOC
CI
CO2CH3
Draw structures of the following compounds and identify their role:
mCPBA
(MCPBA)
DMS
Py
9-BBN
LAH
Sia₂BH
TsCI
PCC
t-BuOK
LDA
MeLi
n-BuLi
DMSO
DMF
Sodium Borohydride
Lithium DiisopropylAmide
2
Using Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode.
DATA: Electrode potentials E° = 0,854 V y E 0,788 V
Hg2+/Hg
2+
Hg2/Hg
Chapter 12 Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
Ch. 12 - Prob. 1PPCh. 12 - Prob. 2PPCh. 12 - Prob. 3PPCh. 12 - PRACTICE PROBLEM 12.4 Predict the products of the...Ch. 12 - Prob. 5PPCh. 12 - Prob. 6PPCh. 12 - Practice Problem 12.7
Provide retrosynthetic...Ch. 12 - Prob. 8PPCh. 12 - What products would you expect from the reaction...Ch. 12 - What products would you expect from the reaction...
Ch. 12 - What product (or products) would be formed from...Ch. 12 - Prob. 12PCh. 12 - 12.13 Write reaction conditions and the product...Ch. 12 - Prob. 14PCh. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the major organic product from each of the...Ch. 12 - Synthesize each of the following compounds from...Ch. 12 - Prob. 20PCh. 12 - 21. Write a mechanism for the following reaction....Ch. 12 - Prob. 22PCh. 12 - 23. What organic products A-H would you expect...Ch. 12 - Prob. 24PCh. 12 - Show how 1-pentanol could be transformed into each...Ch. 12 - Provide the reagents needed to accomplish...Ch. 12 - Prob. 27PCh. 12 - For each of the following alcohols, write a...Ch. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Predict the major organic product from each of the...Ch. 12 - 34. Synthesize the following compound using...Ch. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - 37. Explain how and IR spectroscopy could be used...Ch. 12 - 38. An unknown X shows a broad absorption band in...Ch. 12 - Prob. 39PCh. 12 - The problem below is directed toward devising a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the formation of CaCO3 skeletons by calcareous phytoplankton retard CO2 uptake and help maintain ocean...
Brock Biology of Microorganisms (15th Edition)
9. A pendulum is made by tying a 500 g ball to a 75-cm-long string. The pendulum is pulled 30° to one side, the...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Describe the four curves of the vertebral column.
Principles of Anatomy and Physiology
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
4.55 Using the activity series (Table 4.5), write balanced chemical equations for the following reactions. If ...
Chemistry: The Central Science (14th Edition)
MAKE CONNECTIONS Which chemical group is most likely to be responsible for an organic molecule behaving as a ba...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forwardidentify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forward
- Identify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forwardFor the following compound: HO H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: + H-O: H-OH₂ H Mechanism B: H-Ö: HO-H H-OO -H H HH H H HH H-O: H-OO H-OO -H H e -H : OH Θ Mechanism C: Θ A : OH H-O: H H H-O-H 0. Mechanism D: e.. : OH :0 H H-O-H H-O: H-OO :O H -H H H сём H 0 :0 + H Θ H H H-arrow_forwardFor the following compound: H OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Θ :OH O O-H HO 0: Mechanism B: :O-H e.. Θ :OH Mechanism C: H HO-H :0: Θ 0: H H e.. : OH 0: "Θ HH O. :OH :OH O-H O-H Mechanism D: :OH H-OH₂ :OH HO-H 0: © O-H H HH 0: HHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License