
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
14th Edition
ISBN: 9780136873891
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 21E
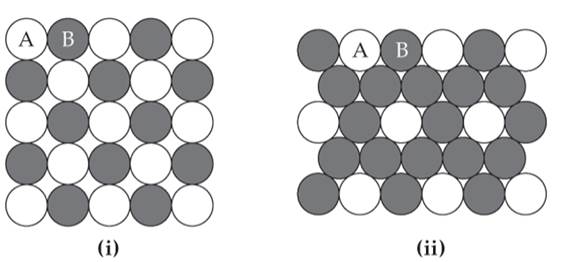
Two patterns of packing for two different circles of the same size are shown here. For each structure
- Draw the two-dimensional unit cell;
- Determine the angle between the lattice vectors , and determine whether the lattice vectors are of the same length or of different lengths; and
- Determine the type of two-dimensional lattice (from Figure 12.4).
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.
If the current voltage is n = 0.14 V, indicate which of the 2 voltage
formulas of the ley of Tafel must be applied
i
a
a) == exp (1-B).
xp[(1 - ß³):
Fn
Fn
a
b) == exp B
RT
RT
If the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied
a)
=
a
T = i exp[(1 - p) F
Fn
Fn
b) i==exp B
RT
Chapter 12 Solutions
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
Ch. 12.3 - Consider the two-dimensional square lattice of...Ch. 12.3 - Prob. 12.1.2PECh. 12.5 - Given the ionic radii and molar masses of Sc3+...Ch. 12.5 - Prob. 12.2.2PECh. 12.7 - Prob. 12.3.1PECh. 12.7 - Prob. 12.3.2PECh. 12.7 - Prob. 12.4.1PECh. 12.7 - Prob. 12.4.2PECh. 12 - Prob. 1DECh. 12 - Prob. 1E
Ch. 12 - Prob. 2ECh. 12 - Prob. 3ECh. 12 - Prob. 4ECh. 12 - Prob. 5ECh. 12 - Prob. 6ECh. 12 - Prob. 7ECh. 12 - Prob. 8ECh. 12 - Prob. 9ECh. 12 - Prob. 10ECh. 12 - 12.11 Covalent bonding occurs in both molecular...Ch. 12 - Prob. 12ECh. 12 - 12.13 What kinds of attractive forces exist...Ch. 12 - Prob. 14ECh. 12 - Prob. 15ECh. 12 - Prob. 16ECh. 12 - Prob. 17ECh. 12 - Prob. 18ECh. 12 - Prob. 19ECh. 12 - Amorphous silica, SiO2, has a density of about...Ch. 12 - Two patterns of packing for two different circles...Ch. 12 - Prob. 22ECh. 12 - Prob. 23ECh. 12 - Prob. 24ECh. 12 - Which of the three-dimensional primitive lattices...Ch. 12 - Prob. 26ECh. 12 - 12.27 What is the minimum number of atoms that...Ch. 12 - 12.28 What is the minimum number of atoms that...Ch. 12 - Prob. 29ECh. 12 - Prob. 30ECh. 12 - Prob. 31ECh. 12 - Prob. 32ECh. 12 - Prob. 33ECh. 12 - Prob. 34ECh. 12 - Prob. 35ECh. 12 - Prob. 36ECh. 12 - Prob. 37ECh. 12 - Prob. 38ECh. 12 - Prob. 39ECh. 12 - Prob. 40ECh. 12 - Prob. 41ECh. 12 - Prob. 42ECh. 12 - Prob. 43ECh. 12 - Prob. 44ECh. 12 - Prob. 45ECh. 12 - Prob. 46ECh. 12 - Prob. 47ECh. 12 - Prob. 48ECh. 12 - Prob. 49ECh. 12 - Prob. 50ECh. 12 - Prob. 51ECh. 12 - Prob. 52ECh. 12 - 12.53 Which would you expect to be the more...Ch. 12 - 12.54 Which of the following statements does not...Ch. 12 - Prob. 55ECh. 12 - Prob. 56ECh. 12 - Prob. 57ECh. 12 - Prob. 58ECh. 12 - Prob. 59ECh. 12 - Prob. 60ECh. 12 - 12.61 A particular form of cinnabar (HgS) adopts...Ch. 12 - At room temperature and pressure RbI crystallizes...Ch. 12 - Prob. 63ECh. 12 - Prob. 64ECh. 12 - The coordination number for Mg2+ ion is usually...Ch. 12 - Prob. 66ECh. 12 - Prob. 67ECh. 12 - Prob. 68ECh. 12 - Prob. 69ECh. 12 - Prob. 70ECh. 12 - Prob. 71ECh. 12 - Prob. 72ECh. 12 - Prob. 73ECh. 12 - Prob. 74ECh. 12 - Prob. 75ECh. 12 - Prob. 76ECh. 12 - Prob. 77ECh. 12 - Prob. 78ECh. 12 - Prob. 79ECh. 12 - Prob. 80ECh. 12 - Prob. 81ECh. 12 - Prob. 82ECh. 12 - Prob. 83ECh. 12 - Prob. 84ECh. 12 - Prob. 85ECh. 12 - 12.86 Write a balanced chemical equation for the...Ch. 12 - Prob. 87ECh. 12 - Prob. 88ECh. 12 - Prob. 89ECh. 12 - Prob. 90ECh. 12 - Prob. 91ECh. 12 - Prob. 92ECh. 12 - Prob. 93ECh. 12 - Prob. 94ECh. 12 - Explain why “bands” may not be the most accurate...Ch. 12 - Prob. 96ECh. 12 - Prob. 97ECh. 12 - Prob. 98ECh. 12 - Prob. 99ECh. 12 - An ideal quantum dot for use in TVs does not...Ch. 12 - Prob. 101ECh. 12 - Prob. 102ECh. 12 - Prob. 103AECh. 12 - Prob. 104AECh. 12 - Prob. 105AECh. 12 - Pure iron crystallizes in a body-centered cubic...Ch. 12 - Prob. 107AECh. 12 - Prob. 108AECh. 12 - Prob. 109AECh. 12 - What type of latticeprimitive cubic, body-centered...Ch. 12 - Prob. 111AECh. 12 - Prob. 112AECh. 12 - Prob. 113AECh. 12 - Energy bands are considered continuous due to the...Ch. 12 - Prob. 115AECh. 12 - Prob. 116AECh. 12 - Prob. 117AECh. 12 - Prob. 118AECh. 12 - Prob. 119AECh. 12 - Prob. 120AECh. 12 - Prob. 121AECh. 12 - Prob. 122AECh. 12 - Prob. 123AECh. 12 - The karat scale used to describe gold alloys is...Ch. 12 - 12.125 Spinel is a mineral that contains 37.9% AI,...Ch. 12 - Prob. 126IECh. 12 - Prob. 127IECh. 12 - Prob. 128IECh. 12 - Prob. 129IECh. 12 - Silicon has the diamond structure with a unit cell...Ch. 12 - Prob. 131IECh. 12 - Prob. 132IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Topic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forward
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY