
College Physics For Ap® Courses
16th Edition
ISBN: 9781938168932
Author: Gregg Wolfe, Irina Lyublinskaya, Douglas Ingram
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 20CQ
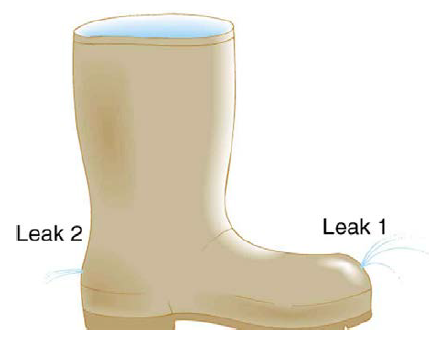
The old rubber boot shown in Figure 12.26 has two leaks. To what maximum height can the water squirt from Leak 1? How does the velocity of water emerging from Leak 2 differ from that of leak 1? Explain your responses in terms of energy.

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
I need help with part B. I cant seem to get the correct answer. Please walk me through what youre doing to get to the answer and what that could be
Question 6:
Chlorine is widely used to purify municipal water supplies and to treat swimming pool
waters. Suppose that the volume of a particular sample of Cl₂ gas is 8.70 L at 895 torr
and 24°C.
(a) How many grams of Cl₂ are in the sample?
⚫ Atomic mass of CI = 35.453 g/mol
• Molar mass of Cl₂ = 2 x 35.453 = 70.906 g/mol
Solution:
Use the Ideal Gas Law:
Step 1: Convert Given Values
• Pressure: P = 895 torr → atm
PV=
= nRT
1
P = 895 ×
= 1.1789 atm
760
•
Temperature: Convert to Kelvin:
T24273.15 = 297.15 K
• Gas constant: R = 0.0821 L atm/mol. K
Volume: V = 8.70 L
Step 2: Solve for n
.
PV
n =
RT
n =
(1.1789)(8.70)
(0.0821)(297.15)
10.25
n =
= 0.420 mol
24.405
Step 3: Calculate Mass of Cl₂
Final Answer: 29.78 g of Cl₂.
mass nx M
mass=
(0.420)(70.906)
mass=
29.78 g
E1
R₁
w
0.50
20 Ω
12
R₁₂
ww
ΒΩ
R₂
60
E3
C
RA
w
15 Ω
E2
0.25
E4
0.75 Ω
0.5 Ω
Chapter 12 Solutions
College Physics For Ap® Courses
Ch. 12 - What is the difference between flow rate and fluid...Ch. 12 - Many figures in the text show streamlines. Explain...Ch. 12 - Identify some substances that are incompressible...Ch. 12 - You can squirt water a considerably greater...Ch. 12 - Water is shot nearly vertically upward in a...Ch. 12 - Look back to Figure 12.4. Answer the following two...Ch. 12 - Give an example of entrainment not mentioned in...Ch. 12 - Many entrainment devices have a constriction,...Ch. 12 - Some chimney pipes have a T-shape, with a...Ch. 12 - Is there a limit to the height to which an...
Ch. 12 - Why is it preferable for airplanes to take off...Ch. 12 - Roofs are sometimes pushed off vertically during a...Ch. 12 - Why does a sailboat need a keel?Ch. 12 - It is dangerous to stand close to railroad tracks...Ch. 12 - Water pressure inside a hose nozzle can be less...Ch. 12 - A perfume bottle or atomizer sprays a fluid that...Ch. 12 - If you lower the window on a car while moving, an...Ch. 12 - Based on Bernoulli's equation, what are three...Ch. 12 - Water that has emerged from a hose into the...Ch. 12 - The old rubber boot shown in Figure 12.26 has two...Ch. 12 - Water pressure inside a hose nozzle can be less...Ch. 12 - Explain why the viscosity of a liquid decreases...Ch. 12 - When paddling a canoe upstream, it is wisest to...Ch. 12 - Why does flow decrease in your shower when someone...Ch. 12 - Plumbing usually includes air-filled tubes near...Ch. 12 - Doppler ultrasound can be used to the speed of...Ch. 12 - Sink drains often have a device such as that shown...Ch. 12 - Some ceiling fans have decorative wicker reeds on...Ch. 12 - What direction will a helium balloon move inside a...Ch. 12 - Will identical raindrops fall more rapidly in 5° C...Ch. 12 - If you took two marbles of different sizes, what...Ch. 12 - Why would you expect the rate of diffusion to...Ch. 12 - How are osmosis and dialysis similar? How do they...Ch. 12 - What is the average flow rate in cm3/S of gasoline...Ch. 12 - The heart of a resting adult pumps blood at a rate...Ch. 12 - Blood is pumped from the heart at a rate of 5.0...Ch. 12 - Blood is flowing through an artery of radius 2 mm...Ch. 12 - The Huka Falls on the Waikato River is one of New...Ch. 12 - A major artery with a cross-sectional area of 1.00...Ch. 12 - (a) As blood passes through the capillary bed in...Ch. 12 - The human circulation system has approximately...Ch. 12 - (a) Estimate the time it would take to fill a...Ch. 12 - The flow rate of blood through 2.00106 -m-radius...Ch. 12 - (a) What is the fluid speed in a fire hose with a...Ch. 12 - The main uptake air duct of a forced air gas...Ch. 12 - Water is moving at a velocity of 2.00 m/s through...Ch. 12 - Prove that the speed of an incompressible fluid...Ch. 12 - Water emerges straight down from a faucet with a...Ch. 12 - Unreasonable Results A mountain stream is 10.0 m...Ch. 12 - Verify that pressure has units of energy per unit...Ch. 12 - Suppose you have a wind speed gauge like the pitot...Ch. 12 - If the pressure reading of your pitot tube is 15.0...Ch. 12 - Calculate the maximum height to which water could...Ch. 12 - Every few years, winds in Boulder, Colorado,...Ch. 12 - (a) Calculate the approximate force on a square...Ch. 12 - (a) What is the pressure drop due to the Bernoulli...Ch. 12 - (a) Using Bernoulli's equation, show that the...Ch. 12 - Hoover Dam on the Colorado River is the highest...Ch. 12 - A frequently quoted rule of thumb in aircraft...Ch. 12 - The left ventricle of a resting adult's heart...Ch. 12 - A sump pump (used to drain water from the basement...Ch. 12 - (a) Calculate the retarding force due to the...Ch. 12 - What force is needed to pull one microscope slide...Ch. 12 - A glucose solution being administered with an IV...Ch. 12 - The pressure drop along a length of artery is 100...Ch. 12 - A small artery has a length of 1.1103 m and a...Ch. 12 - Fluid originally flows through a tube at a rate of...Ch. 12 - The arterioles (small arteries) leading to an...Ch. 12 - Angioplasty is a technique in which arteries...Ch. 12 - (a) Suppose a blood vessel's radius is decreased...Ch. 12 - A spherical particle falling at a terminal speed...Ch. 12 - Using the equation of the previous problem, find...Ch. 12 - A skydiver will reach a terminal velocity when the...Ch. 12 - A layer of oil 1.50 mm thick is placed between two...Ch. 12 - (a) Verify that a 19.0% decrease in laminar flow...Ch. 12 - Example 12.8 dealt with the flow of saline...Ch. 12 - When physicians diagnose arterial blockages, they...Ch. 12 - During a marathon race, a runner's blood flow...Ch. 12 - Water supplied to a house by a water main has a...Ch. 12 - An oil gusher shoots crude oil 25.0 m into the air...Ch. 12 - Concrete is pumped from a cement mixer to the...Ch. 12 - Construct Your Own Problem Consider a coronary...Ch. 12 - Consider a river that spreads out in a delta...Ch. 12 - Verify that the flow of oil is laminar (barely)...Ch. 12 - Show that the Reynolds number NRis unitless by...Ch. 12 - Calculate the Reynolds numbers for the flow of...Ch. 12 - A fire hose has an inside diameter of 6.40 cm....Ch. 12 - Concrete is pumped from a cement mixer to the...Ch. 12 - At what flow rate might turbulence begin to...Ch. 12 - What is the greatest average speed of blood flow...Ch. 12 - In Take-Home Experiment: Inhalation, we measured...Ch. 12 - Gasoline is piped underground from refineries to...Ch. 12 - Assuming that blood is an ideal fluid, calculate...Ch. 12 - Unreasonable Results A fairly large garden hose...Ch. 12 - You can smell perfume very shortly after opening...Ch. 12 - What is the ratio of the average distances that...Ch. 12 - Oxygen reaches the veinless cornea of the eye by...Ch. 12 - (a) Find the average time required for an oxygen...Ch. 12 - Suppose hydrogen and oxygen are diffusing through...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
Match the substance on the left with the basic units that compose them on the right Remember that atomic elemen...
Introductory Chemistry (6th Edition)
Body, Heal Thyself The precision of mitotic cell division is essential for repairing damaged tissues like those...
Biology: Life on Earth with Physiology (11th Edition)
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
16. On the Apollo 14 mission to the moon, astronaut Alan Shepard hit a golf ball with a 6 iron. The free-fall a...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the force (in N) on the 2.0 μC charge placed at the center of the square shown below? (Express your answer in vector form.) 5.0 με 4.0 με 2.0 με + 1.0 m 1.0 m -40 με 2.0 μCarrow_forwardWhat is the force (in N) on the 5.4 µC charge shown below? (Express your answer in vector form.) −3.1 µC5.4 µC9.2 µC6.4 µCarrow_forwardAn ideal gas in a sealed container starts out at a pressure of 8900 N/m2 and a volume of 5.7 m3. If the gas expands to a volume of 6.3 m3 while the pressure is held constant (still at 8900 N/m2), how much work is done by the gas? Give your answer as the number of Joules.arrow_forward
- The outside temperature is 25 °C. A heat engine operates in the environment (Tc = 25 °C) at 50% efficiency. How hot does it need to get the high temperature up to in Celsius?arrow_forwardGas is compressed in a cylinder creating 31 Joules of work on the gas during the isothermal process. How much heat flows from the gas into the cylinder in Joules?arrow_forwardThe heat engine gives 1100 Joules of energy of high temperature from the burning gasoline by exhausting 750 Joules to low-temperature . What is the efficiency of this heat engine in a percentage?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill
Fluids in Motion: Crash Course Physics #15; Author: Crash Course;https://www.youtube.com/watch?v=fJefjG3xhW0;License: Standard YouTube License, CC-BY