
(a)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
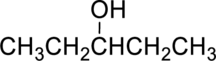
(a)
Explanation of Solution
The given alcohol is,
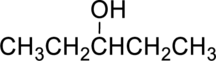
Parent compound : pentane
Position of –OH : carbon – 3
Substituent : No substituent present
IUPAC Name : pentan-3-ol
Therefore, the
Pentan-3-ol is a secondary alcohol it undergoes oxidation reaction to produce 3-pentanone. The reaction can be shown as follows,
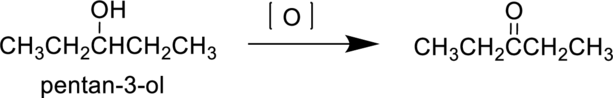
Parent compound : pentane
Position of
Substituent : No substituent present
IUPAC Name : pentan-3-one
Therefore, the IUPAC nomenclature for the given product is pentan-3-one.
(b)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
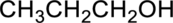
(b)
Explanation of Solution
The given alcohol is,
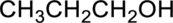
Parent compound : propane
Position of –OH : carbon – 1
Substituent : No substituent present
IUPAC Name : propan-1-ol
Therefore, the IUPAC nomenclature for the given alcohol is propan-1-ol.
Propan-1-ol is a primary alcohol it undergoes oxidation reaction to produce propanal and forms propanoic acid on further oxidation. The reaction can be shown as follows,
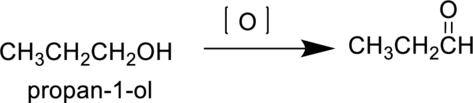
Parent compound : propane
Position of
Substituent : No substituent present
IUPAC Name : propan-1-al
Therefore, the IUPAC nomenclature for the given product is propan-1-al.
(c)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
(c)
Explanation of Solution
The given alcohol is,
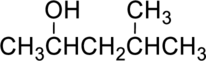
Parent compound : pentane
Position of –OH : carbon – 2
Substituent : 4-methyl
IUPAC Name : 4-methylpentan-2-ol
Therefore, the IUPAC nomenclature for the given alcohol is 4-methylpentan-2-ol.
4-methylpentan-2-ol is a secondary alcohol it undergoes oxidation reaction to produce 4-methylpentan-2-one. The reaction can be shown as follows,
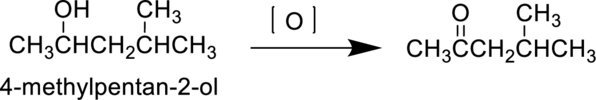
Parent compound : pentane
Position of
Substituent : 4-methyl
IUPAC Name : 4-methylpentan-2-one
Therefore, the IUPAC nomenclature for the given product is 4-methylpentan-2-one.
(d)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
(d)
Explanation of Solution
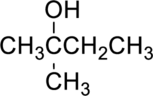
The given alcohol is,
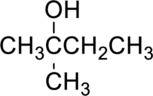
Parent compound : butane
Position of –OH : carbon – 2
Substituent : 2-methyl
IUPAC Name : 2-methylbutan-2-ol
Therefore, the IUPAC nomenclature for the given alcohol is 2-methylbutan-2-ol.
2-methylbutan-2-ol is a tertiary alcohol it does not undergoes oxidation reaction. Hence the it considered as N.R and it can be shown as follows,
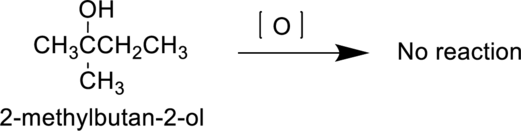
(e)
Interpretation:
The IUPAC name for the given alcohol and its oxidation product has to be written.
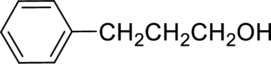
(e)
Explanation of Solution
The given alcohol is,
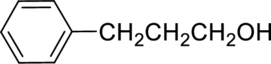
Parent compound : propane
Position of –OH : carbon – 1
Substituent : 3-phenyl
IUPAC Name : 3-phenylpropan-1-ol
Therefore, the IUPAC nomenclature for the given alcohol is 3-phenylpropan-1-ol.
3-phenylpropan-1-ol is a primary alcohol it undergoes oxidation reaction to produce 3-phenylpropan-1-al and forms 3-phenylpropanoic acid on further oxidation. The reaction can be shown as follows,
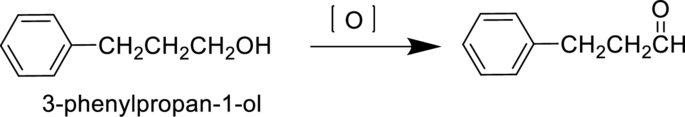
Parent compound : propane
Position of
Substituent : 3-phenyl
IUPAC Name : 3-phenylpropan-1-al
Therefore, the IUPAC nomenclature for the given product is 3-phenylpropan-1-al.
Want to see more full solutions like this?
Chapter 12 Solutions
GENERAL,ORGANIC+BIOCHEM (LOOSELEAF)
- Identify and provide a concise explanation of the concept of signal-to-noise ratio (SNR) in the context of chemical analysis. Provide specific examples.arrow_forwardIdentify and provide a concise explanation of a specific analytical instrument capable of detecting and quantifying trace compounds in food samples. Emphasise the instrumental capabilities relevant to trace compound analysis in the nominated food. Include the specific application name (eg: identification and quantification of mercury in salmon), outline a brief description of sample preparation procedures, and provide a summary of the obtained results from the analytical process.arrow_forwardIdentify and provide an explanation of what 'Seperation Science' is. Also describe its importance with the respect to the chemical analysis of food. Provide specific examples.arrow_forward
- 5. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn. H3C CH3arrow_forwardState the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardState the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forward
- Provide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardGiven a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forward
- The molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forwardIn GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





