
Student's Solutions Manual for Organic Chemistry
9th Edition
ISBN: 9780134160375
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.31SP
Consider the following four structures, followed by mass spectral data. Match each structure with its characteristic molecular ion or fragment. In each case, give a likely structure of the ion responsible for the base peak.
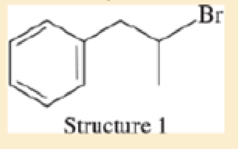
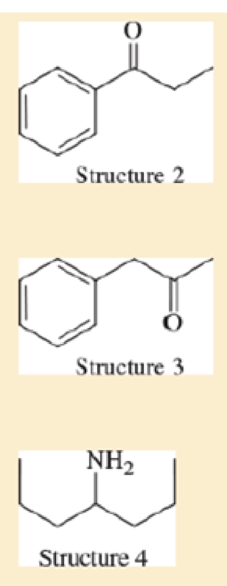
- a. base peak at 105
- b. base peak at 72
- c. M+ doublet at 198 and 200, base peak at 91
- d. base peak at 91, large peak at 43
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.
At a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?
Topic: Photochemistry and Photophysics of Supramolecules
Chapter 12 Solutions
Student's Solutions Manual for Organic Chemistry
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Give the IUPAC name for each compound.
Organic Chemistry
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forwardCalculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forward
- The decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forward
- Indicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forwardHow can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY