
Concept explainers
MCAT-Style Passage Problems
Thermal Properties of the Oceans
Seasonal temperature changes in the ocean only affect the top layer of water, to a depth of 500 0m or so. This “mixed” layer is thermally isolated from the cold, deep water below. The average temperature of this top layer of the world’s oceans, which has area 3.6 × 108 km2, is approximately 17°C.
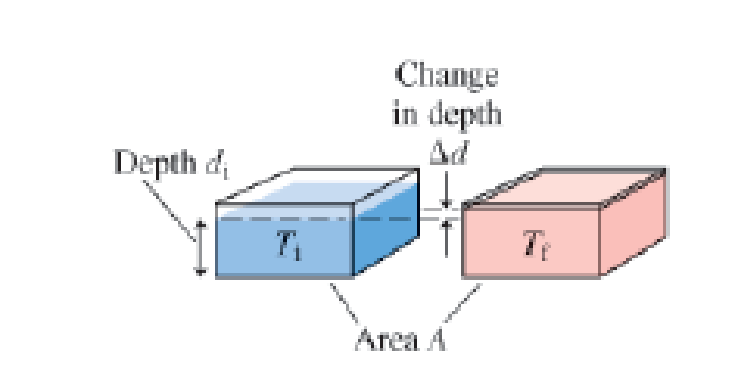
In addition to seasonal temperature changes, the oceans have experienced an overall warming trend over the last century that is expected to continue as the earth’s climate changes. A warmer ocean means a larger volume of water; the oceans will rise. Suppose the average temperature of the top layer of the world's oceans were to increase from a temperature Ti; to a temperature Tf. The area of the oceans will not change, as this is fixed by the size of the ocean basin, so any thermal expansion of the water will cause the water level to rise, as shown in Figure P12.109. The original volume is the product of the original depth and the surface area, Vi = Adi. The change in volume is given by ΔV = A Δd.
Figure P12.109
The ocean is mostly heated from the top, by light from the sun. The warmer surface water doesn’t mix much with the colder deep ocean water. This lack of mixing can be ascribed to a lack of
A.
B.
C.
D. Evaporation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Mastering Physics with Pearson eText -- Standalone Access Card -- for College Physics: A Strategic Approach (3rd Edition)
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Human Physiology: An Integrated Approach (8th Edition)
Human Anatomy & Physiology (2nd Edition)
Organic Chemistry (8th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- The character Min Min from Arms was a DLC character added to Super Smash Bros. Min Min’s arms are large springs, with a spring constant of 8.53 ⋅ 10^3 N/m, which she uses to punch and fling away her opponents. Min Min pushes her spring arm against Steve, who is not moving, compressing it 1.20 m as shown in figure A. Steve has a mass of 81.6 kg. Assuming she uses only the spring to launch Steve, how fast is Steve moving when the spring is no longer compressed? As Steve goes flying away he goes over the edge of the level, as shown in figure C. What is the magnitude of Steve’s velocity when he is 2.00 m below where he started?arrow_forwardCalculate the energy needed to melt 50 g of 0°C icearrow_forwardTwo very long line charges are set up along lines that areparallel to the z-axis, so they set up Electric fields strictly in the xy plane. One goes throughthe x-axis at x = −0.40 m and has charge a density λ1 = +12.0 μC/m, the other goesthrough the x-axis at x = +0.40 m has charge density λ2 = −8.0 μC/m.A. Find the Electric field at point A: (0.40, 0.80) (distances in meters). Give answersin unit vector notation and draw a graph of the x-y plane with the E-fields you justfound.B. Find a point on the x-axis at which the total E-field is 0.arrow_forward
- In order to increase the amount of exercise in her daily routine, Tara decides to walk up the four flights of stairs to her car instead of taking the elevator. Each of the steps she takes are 18.0 cm high, and there are 12 steps per flight. (a) If Tara has a mass of 77.0 kg, what is the change in the gravitational potential energy of the Tara-Earth system (in J) when she reaches her car? ] (b) If the human body burns 1.5 Calories (6.28 x 10³ J) for each ten steps climbed, how much energy (in J) has Tara burned during her climb? ] (c) How does the energy she burned compare to the change in the gravitational potential energy of the system? Eburned Δυarrow_forwardA 4.40 kg steel ball is dropped onto a copper plate from a height of 10.0 m. If the ball leaves a dent 2.75 mm deep, what is the average force exerted by the plate on the ball during the impact? Narrow_forwardA block of mass m = 7.00 kg is released from rest from point and slides on the frictionless track shown in the figure below. (Assume h₂ = 7.80 m.) a m ha 3.20 m 2.00 m i (a) Determine the block's speed at points ® and point B ©. m/s m/s point (b) Determine the net work done by the gravitational force on the block as it moves from point J A to pointarrow_forward
- A 1.10 x 10²-g particle is released from rest at point A on the inside of a smooth hemispherical bowl of radius R R B 2R/3 (a) Calculate its gravitational potential energy at A relative to B. ] (b) Calculate its kinetic energy at B. ] (c) Calculate its speed at B. m/s (d) Calculate its potential energy at C relative to B. J (e) Calculate its kinetic energy at C. ] = 26.5 cm (figure below).arrow_forwardReport on the percentage errors (with uncertainty) between the value of 'k' from the F vs displacement plot and each of the values of 'k' from the period measurements. Please comment on the goodness of the results. Value of k = Spring constant k = 50.00 N/m Each of the values of k from period measurements: Six Measurements of time for 5 osccilations: t1 = 7.76s, t2=8.00s, t3=7.40s, t4=7.00s, t5=6.90s, t6=7.10s (t1-tavg)^2 = (7.76-7.36)^2 = 0.16%(t2-tavg)^2 =(8.00-7.36)^2 = 0.4096%(t3-tavg)^2 =(7.40-7.36)^2 = 0.0016%(t4-tavg)^2 =(7.00-7.36)^2 = 0.1296%(t5-tavg)^2 =(6.90-7.36)^2 = 0.2116%(t6-tavg)^2 =(7.10-7.36)^2 = 0.0676arrow_forwardNo chatgpt pls will upvotearrow_forward
- Based on the two periods (from hand timed and ultrasonic sensor), find the value of 'k' they suggest from the physics and from the value of the hanging mass. hand time period is 1.472s and ultrasonic sensor time period is 1.44sarrow_forwardNo chatgpt pls will upvotearrow_forwardExperimental Research Report Template Title: Paper Airplane Flight. Materials: Paper, ruler, tape Procedure: Fold paper into different airplane designs, such as dart, glider, or classic. Measure and record the distances each design flies when thrown with the same force. Discuss aerodynamics and the factors that affect flight distance. Introduction: (What do you expect to learn? What is the purpose of this lab? List any questions this experiment will answer.) Hypothesis: (Predict the outcome(s) of the experiment, must be in an “if…then format.) Materials: (What equipment and materials did you need for this experiment assignment? Describe how any equipment was connected. Also mention any special hardware or connections. List the name and amount of each item used.) Procedures: (What steps did you take to accomplish this lab assignment? Include Safety Precautions.) Data Collection: (Record the data that is required at each step of the…arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
 College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





