
Concept explainers
Chemical/Bio Engineering
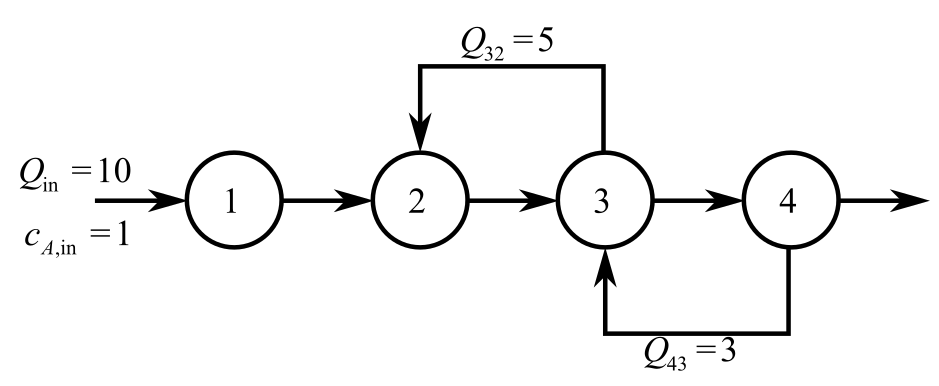
An irreversible, first-order reaction takes place in four well mixed reactors (Fig. P12.10),
Thus, the rate at which A is transformed to B can be represented as
The reactors have different volumes, and because they are operated at different temperatures, each has a different reaction rate:
| Reactor | V, L |
|
| 1 | 25 | 0.05 |
| 2 | 75 | 0.1 |
| 3 | 100 | 0.5 |
| 4 | 25 | 0.1 |
Determine the concentration of A and B in each of the reactors at steady state.
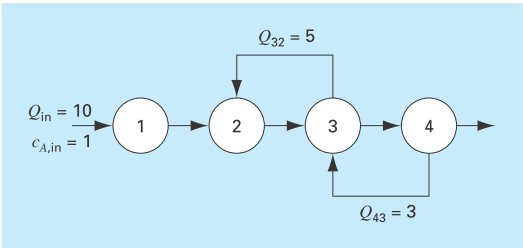
FIGURE P12.10
To calculate:
Answer to Problem 10P
Solution:
Explanation of Solution
Given Information:
Write the provided values of the volume and rate of reaction.
Formula used:
Write system of linear equations in matrix form.
And,
The term
Calculation:
Consider the provided diagram for an irreversible first order reaction takes place in four will-mixed reactors.
Balance the mass for A in reactor 1 at steady-state.
Substitute
Further solve.
Balance the mass for A in reactor 2 at steady-state.
Substitute
Further solve.
Balance the mass for A in reactor 3 at steady-state.
Substitute
Further solve.
Balance the mass for A in reactor 4 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 1 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 2 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 3 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 4 at steady-state.
Substitute
Further solve.
Now, write all the equations, to find linear system of equations.
Write the above linear equations in matrix form as written in symbolized form.
Here, coefficient matrix A is,
Column matrix
Column matrix B is,
Substitute the values in the matrix equation form.
Solve for
Code:
Type the above code into MATLAB command window and press enter to find the result.
Result is obtained as follows:
Hence,
Hence, the concentration of A and B is,
Want to see more full solutions like this?
Chapter 12 Solutions
Numerical Methods For Engineers, 7 Ed
Additional Math Textbook Solutions
Precalculus: Mathematics for Calculus (Standalone Book)
Elementary Statistics ( 3rd International Edition ) Isbn:9781260092561
Precalculus
Intermediate Algebra (13th Edition)
- No chatgpt plsarrow_forwardcan you help me solve the parts and show workings pleasearrow_forwardSuppose that a room containing 1300 cubic feet of air is originally free of carbon monoxide (CO). Beginning at time t = 0, cigarette smoke containing 4% CO is introduced into the room at a rate of 0.8 cubic feet per minute. The well-circulated smoke and air mixture is allowed to leave the room at the same rate. Let A(t) represent the amount of CO in the room (in cubic feet) after t minutes. (A) Write the DE model for the time rate of change of CO in the room. Also state the initial condition. dA dt A(0) (B) Solve the IVP to find the amount of CO in the room at any time t > 0. A(t) (C) Extended exposure to a CO concentration as low as 0.00012 is harmful to the human body. Find the time at which this concentration is reached. t= minutesarrow_forward
- You buy a house for $210000, and take out a 30-year mortgage at 7% interest. For simplicity, assume that interest compounds continuously. A) What will be your annual mortgage payment? $ per year B) Suppose that regular raises at your job allow you to increase your annual payment by 6% each year. For simplicity, assume this is a nominal rate, and your payment amount increases continuously. How long will it take to pay off the mortgage? yearsarrow_forwardYour employer automatically puts 5 percent of your salary into a 401(k) retirement account each year. The account earns 8% interest. Suppose you just got the job, your starting salary is $40000, and you expect to receive a 2% raise each year. For simplicity, assume that interest earned and your raises are given as nominal rates and compound continuously. Find the value of your retirement account after 30 years Value = $arrow_forwardSuppose that a room containing 1300 cubic feet of air is originally free of carbon monoxide (CO). Beginning at time t = 0, cigarette smoke containing 4% CO is introduced into the room at a rate of 0.8 cubic feet per minute. The well-circulated smoke and air mixture is allowed to leave the room at the same rate. Let A(t) represent the amount of CO in the room (in cubic feet) after t minutes. (A) Write the DE model for the time rate of change of CO in the room. Also state the initial condition. dA dt A(0) (B) Solve the IVP to find the amount of CO in the room at any time t > 0. A(t) (C) Extended exposure to a CO concentration as low as 0.00012 is harmful to the human body. Find the time at which this concentration is reached. t= minutesarrow_forward
- Newton's Law of Cooling tells us that the rate of change of the temperature of an object is proportional to the temperature difference between the object and its surroundings. This can be modeled by the differential equation dT dt k(TA), where T is the temperature of the object after t units of time have passed, A is the ambient temperature of the object's surroundings, and k is a constant of proportionality. Suppose that a cup of coffee begins at 178 degrees and, after sitting in room temperature of 61 degrees for 12 minutes, the coffee reaches 171 degrees. How long will it take before the coffee reaches 155 degrees? Include at least 2 decimal places in your answer. minutesarrow_forwardcan you help me solve this question and show workings pleasearrow_forwardLet f : X → Y and g : Y → Z be two functions. Prove that(1) if g ◦ f is injective, then f is injective; (2) if g ◦ f is surjective, then g is surjective.arrow_forward
- Algebra & Trigonometry with Analytic GeometryAlgebraISBN:9781133382119Author:SwokowskiPublisher:Cengage
 Functions and Change: A Modeling Approach to Coll...AlgebraISBN:9781337111348Author:Bruce Crauder, Benny Evans, Alan NoellPublisher:Cengage Learning
Functions and Change: A Modeling Approach to Coll...AlgebraISBN:9781337111348Author:Bruce Crauder, Benny Evans, Alan NoellPublisher:Cengage Learning Linear Algebra: A Modern IntroductionAlgebraISBN:9781285463247Author:David PoolePublisher:Cengage Learning
Linear Algebra: A Modern IntroductionAlgebraISBN:9781285463247Author:David PoolePublisher:Cengage Learning


