
a) NaBH4 and H3O+
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated first with NaBH4 and then with H3O+ are to be given.
Concept introduction:
NaBH4, either in water or in alcohol reduce
To give:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated first with NaBH4 and then with H3O+.
Answer to Problem 58AP
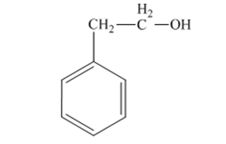
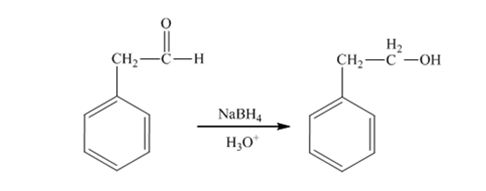
The product produced when 1) phenylacetaldehyde is treated first with NaBH4 and then with H3O+ is 2-phenylethanol.
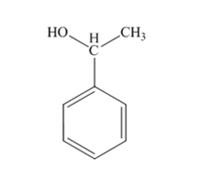
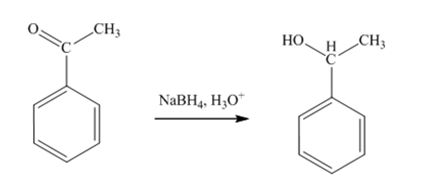
The product produced when acetophenone is treated first with NaBH4 and then with H3O+ is 1-phenylethanol.
Explanation of Solution
Phenylacetaldehyde, being an aldehyde is reduced by NaBH4 to a primary alcohol, 2-phenylethanol.
Acetophenone, being a ketone is reduced by NaBH4 to a secondary alcohol, 1-phenylethanol.
The product produced when 1) phenylacetaldehyde is treated first with NaBH4 and then with H3O+ is 2-phenylethanol.

The product produced when acetophenone is treated first with NaBH4 and then with H3O+ is 1-phenylethanol.

b) Dess-Martin reagent
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with Dess-Martin reagent are to be given.
Concept introduction:
Aldehydes and ketones are not oxidized by Dess-Martin reagent.
To give:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with Dess-Martin reagent.
Answer to Problem 58AP
Phenylacetaldehyde does not react with Dess-Martin reagent.
Acetophenone does not react with Dess-Martin reagent.
Explanation of Solution
Aldehydes and ketones are not oxidized by Dess-Martin reagent.
Phenylacetaldehyde does not react with Dess-Martin reagent.
Acetophenone does not react with Dess-Martin reagent.
c) NH2OH, HCl catalyst
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with NH2OH in the presence of HCl catalyst are to be given.
Concept introduction:
Aldehydes and ketones react with hydroxyl amne in the presence of an acid catalyst to yield aloximes and ketoximes respectively.
To give:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with NH2OH in the presence of HCl catalyst.
Answer to Problem 58AP
The product produced when phenylacetaldehyde is treated with NH2OH in the presence of HCl catalyst is
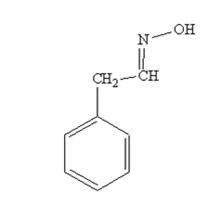
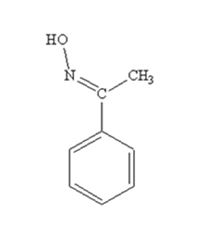
The product produced when acetophenone is treated with NH2OH in the presence of HCl catalyst is
Explanation of Solution
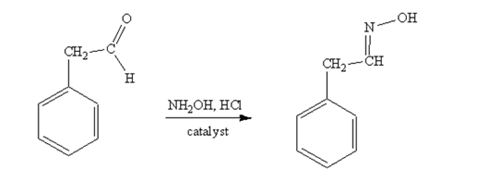
phenylacetaldehyde reacts with NH2OH in the presence of HCl catalystto yield phenylacetaldoxime.
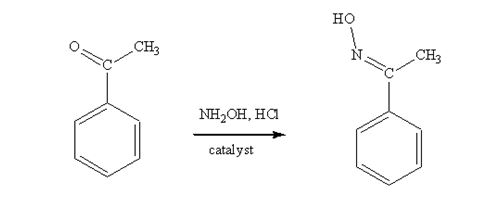
Acetophenone reacts with NH2OH in the presence of HCl catalyst to give acetophenoneoxime.
The product produced when phenylacetaldehyde is treated with NH2OH in the presence of HCl catalyst is

The product produced when acetophenone is treated with NH2OH in the presence of HCl catalyst is

d) CH3MgBr and H3O+
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated first with CH3MgBr and then with H3O+ are to be given.
Concept introduction:
On treatment with Grignard reagents followed by acidification, aldehydes other than formaldehyde yield secondary alcohols and ketones give tertiary alcohols.
To give:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated first with CH3MgBr and then with H3O+.
Answer to Problem 58AP
The product produced when phenylacetaldehyde is treated first with CH3MgBr and then with H3O+ is
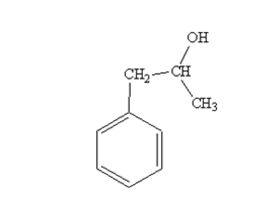
The products produced when acetophenone is treated first with CH3MgBr and then with H3O+ is
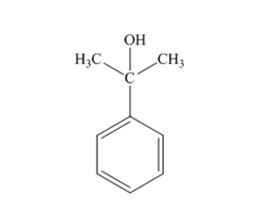
Explanation of Solution
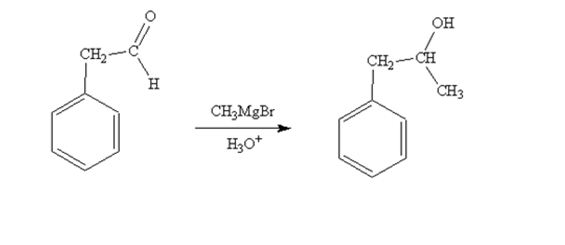
When phenylacetaldehyde is treated first with CH3MgBr and then with H3O+ the product produced is 1-phenyl-2-propanol, a secondary alcohol.
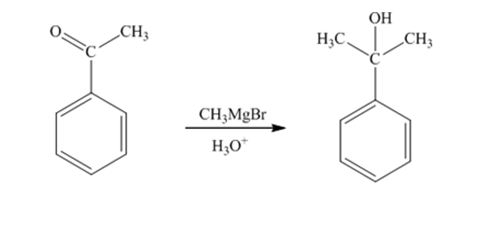
When acetophenone is treated first with CH3MgBr and then with H3O+ the product produced is 2-phenyl-2-propanol, a tertiary alcohol.
The product produced when phenylacetaldehyde is treated first with CH3MgBr and then with H3O+ is

The products produced when acetophenone is treated first with CH3MgBr and then with H3O+ is

e) CH3OH, HCl catalyst
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with two equivalents of CH3OH in the presence of HCl catalyst are to be given.
Concept introduction:
Aldehydes react reversibley with two equivalents of an alcohol in the presence of an acid catalyst to yield acetals and ketones react to give ketals.
To give:
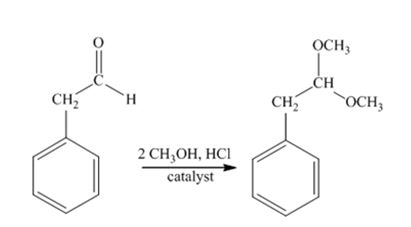
The product produced when phenylacetaldehyde is treated with two equivalents of CH3OH in the presence of HCl catalyst.
Answer to Problem 58AP
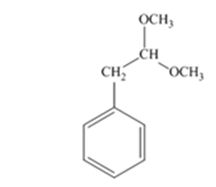
The product produced when phenylacetaldehyde is treated with two equivalents of CH3OH in the presence of HCl catalyst is
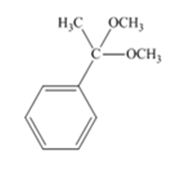
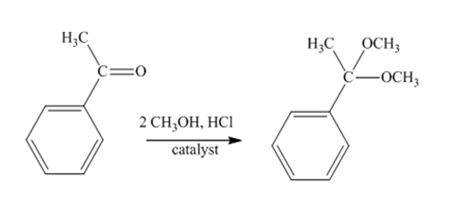
The product produced when acetophenone is treated with two equivalents of CH3OH in the presence of HCl catalyst is
Explanation of Solution
Phenylacetaldehyde reacts with two equivalents of CH3OH in the presence of HCl catalyst to yield an acetal.
Acetophenone reacts with two equivalents of CH3OH in the presence of HCl catalyst to yield a ketal.
The product produced when phenylacetaldehyde is treated with two equivalents of CH3OH in the presence of HCl catalyst is

The product produced when acetophenone is treated with two equivalents of CH3OH in the presence of HCl catalyst is

f) NH2NH2, KOH
Interpretation:
The product produced when 1) phenylacetaldehyde and 2) acetophenone are treated with NH2NH2 in the presence of KOH are to be given.
Concept introduction:
The aldehydes and ketones undergo Wolff-Kishner reduction to yield
To give:
The product produced when 1) phenylacetaldehyde and 2) acetophenone are treated first with NH2NH2 and KOH.
Answer to Problem 58AP
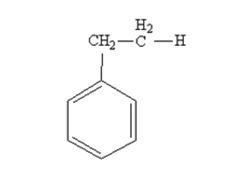
The product produced when phenylacetaldehyde is treated with NH2NH2 and KOH is
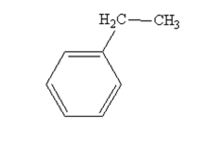
The product produced when acetophenone is treated with NH2NH2 and KOH is
Explanation of Solution
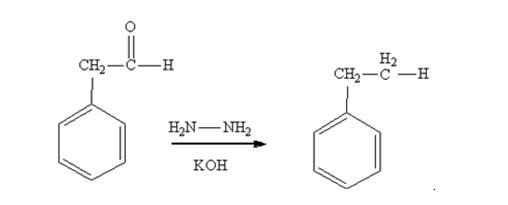
Phenylacetaldehyde is reduced by NH2NH2 and KOH to ethylbenzene.
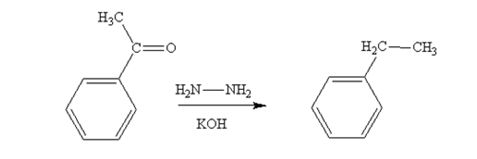
Acetophenone also is reduced by NH2NH2 and KOH to ethylbenzene.
The product produced when phenylacetaldehyde is treated with NH2NH2 and KOH is

The product produced when acetophenone is treated with NH2NH2 and KOH is

g) (C6H5)3P=CH2
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with (C6H5)3P=CH2 are to be given.
Concept introduction:
The reaction given is Wittig reaction. Aldehydes and ketones are converted into
To give:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with (C6H5)3P=CH2.
Answer to Problem 58AP
The product produced when phenylacetaldehyde is treated with (C6H5)3P=CH2 is
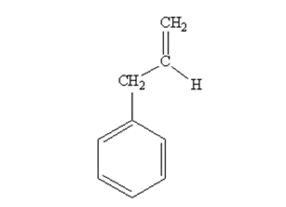
The product produced when acetophenone are treated with (C6H5)3P=CH2 is
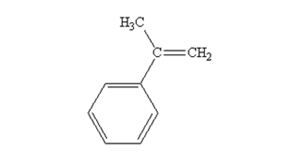
Explanation of Solution
Phenylacetaldehyde reacts with with (C6H5)3P=CH2 to yield 3-phenylpropene.
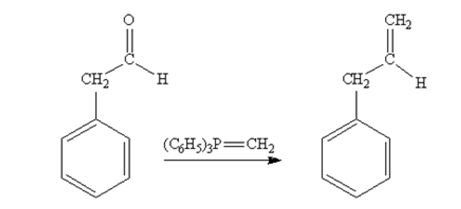
Acetophenone reacts with with (C6H5)3P=CH2 to yield 2-phenylpropene.
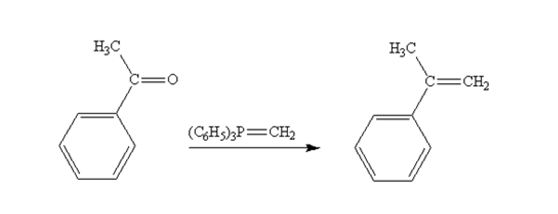
The product produced when phenylacetaldehyde is treated with (C6H5)3P=CH2 is

The product produced when acetophenone are treated with (C6H5)3P=CH2 is

h) HCN, KCN
Interpretation:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated with HCN, KCN are to be given.
Concept introduction:
Aldehydes and ketones undergo nucleophilic addition reaction.
To give:
The products produced when 1) phenylacetaldehyde and 2) acetophenone are treated first with HCN, KCN to yield their cyanohydrins.
Answer to Problem 58AP
The product produced when phenylacetaldehyde is treated with HCN, KCN is
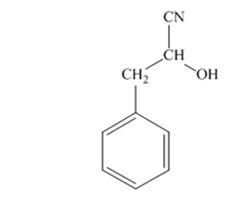
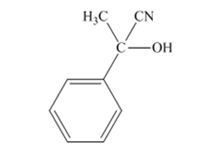
The products produced when acetophenone is treated with HCN, KCN is
Explanation of Solution
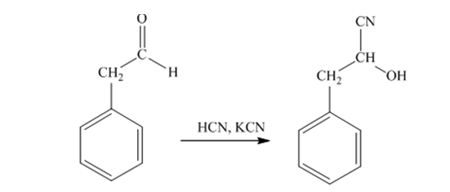
When phenylacetaldehyde is treated with HCN, KCN, it gives phenylacetaldehydecyanohydrin.
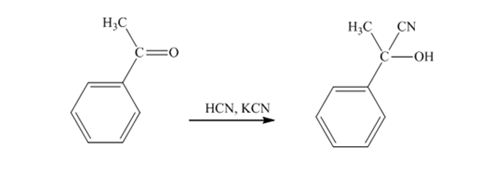
When acetophenone is treated with HCN, KCN, it gives acetophenonecyanohydrin.
The product produced when phenylacetaldehyde is treated with HCN, KCN is

The products produced when acetophenone is treated with HCN, KCN is

Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- identify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forward
- Is (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forwardThree pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forward
- Draw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


