
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
8th Edition
ISBN: 9781337687539
Author: Brown/Iverson/Anslyn/ Foote
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.9, Problem DQ
Interpretation Introduction
Interpretation:
The relative amount of trans-tetrol to cis-tetrol at
Concept Introduction:
Ring opening: Ring opening of epoxide takes place by acid-catalyzed hydrolysis of an epoxide.
Acid-catalyzed hydrolysis of an epoxide via
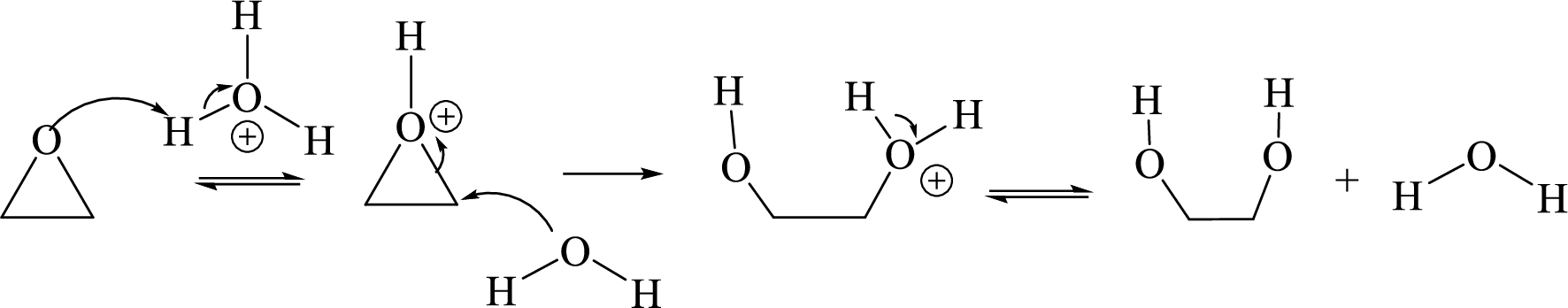
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the major product of this reaction. Ignore inorganic byproducts.
Assume that the water side product is continuously removed to drive the reaction toward products.
O
CH3CH2NH2, TSOH
Select to Draw
>
Predict the major organic product(s) for the following reaction.
Predict the major organic product(s) for the following reactions.
Chapter 11 Solutions
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Knowledge Booster
Similar questions
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
- If I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forward
- If I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning