
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
8th Edition
ISBN: 2818440043505
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.3, Problem 7P
(a)
Interpretation Introduction
Interpretation:
The bromo-substituted compound required to form the following compound has to be identified.
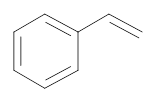
Concept introduction:
Dialkyl lithium cuprate :
(b)
Interpretation Introduction
Interpretation:
The bromo-substituted compound required for the synthesis of the following compound has to be determined.
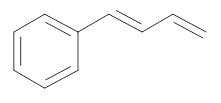
Concept introduction:
Dialkyl lithium cuprate :
(c)
Interpretation Introduction
Interpretation:
The bromo-substituted compound should be determined for the following compound.
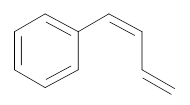
Concept introduction:
Dialkyl lithium cuprate :
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
my ccc edu - Search
X
Quick Access
X
D2L Homepage - Spring 2025 x N Netflix
X
Dimensional Analysis - A x+
pp.aktiv.com
Q ☆
X
Question 59 of 70
The volume of
1
unit of plasma is 200.0 mL
If the recommended dosage
for adult patients is 10.0 mL per kg of body mass, how many units are needed for
a patient with a body mass of 80.0
kg ?
80.0
kg
10.0
DAL
1
units
X
X
4.00
units
1
1
Jeg
200.0
DAL
L
1 units
X
200.0 mL
= 4.00 units
ADD FACTOR
*( )
DELETE
ANSWER
RESET
D
200.0
2.00
1.60 × 10³
80.0
4.00
0.0400
0.250
10.0
8.00
&
mL
mL/kg
kg
units/mL
L
unit
Q Search
delete
prt sc
111
110
19
Identify the starting material in the following reaction. Click the "draw structure" button to launch the
drawing utility.
draw structure ...
[1] 0 3
C10H18
[2] CH3SCH3
H
In an equilibrium mixture of the formation of ammonia from nitrogen and hydrogen, it is found that
PNH3 = 0.147 atm, PN2 = 1.41 atm and Pн2 = 6.00 atm. Evaluate Kp and Kc at 500 °C.
2 NH3 (g) N2 (g) + 3 H₂ (g)
K₂ = (PN2)(PH2)³ = (1.41) (6.00)³ = 1.41 x 104
Chapter 11 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
Ch. 11.1 - Prob. 1PCh. 11.2 - Which is more reactive an organolithium compound...Ch. 11.2 - Prob. 3PCh. 11.3 - Muscalure is the sex attractant of the common...Ch. 11.3 - Prob. 7PCh. 11.3 - Prob. 8PCh. 11.3 - Prob. 9PCh. 11.3 - Prob. 10PCh. 11.4 - Prob. 13PCh. 11.4 - Prob. 14P
Ch. 11.4 - Prob. 15PCh. 11.4 - Prob. 16PCh. 11.4 - Prob. 17PCh. 11.4 - Prob. 19PCh. 11.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 11.4 - Identify two pairs of an alkyl bromide and an...Ch. 11.5 - Prob. 22PCh. 11.5 - Draw the product of ring-closing metathesis for...Ch. 11.5 - Prob. 25PCh. 11.5 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - The coupling of an alkyne with an aryl halide in...Ch. 11 - Identify A through H.Ch. 11 - Using the given starting material, any necessary...Ch. 11 - What alkyl halide reacts with lithium...Ch. 11 - Prob. 33PCh. 11 - Prob. 34PCh. 11 - The following compound undergoes an intramolecular...Ch. 11 - Using ethynyleyclohexane as a starting material...Ch. 11 - Prob. 37PCh. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 39PCh. 11 - A student added an equivalent of...Ch. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 42PCh. 11 - Prob. 43PCh. 11 - Bombykol is the sex pheromone of the silk moth....Ch. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - A dibromide loses only one bromine when it reacts...Ch. 11 - What starting material is required in order to...Ch. 11 - What product is obtained from ring-opening...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forward
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co