
Concept explainers
(a)
Interpretation:
The shape around the labelled atoms needs to be determined.
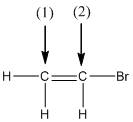
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of lone pairs | Shape | Bond angle | |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
(b)
Interpretation:
The shape around the labelled atoms needs to be determined.
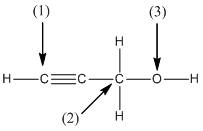
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
(c)
Interpretation:
The shape around the labelled atoms needs to be determined.
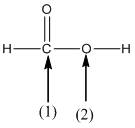
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
(d)
Interpretation:
The shape around the labelled atoms needs to be determined.
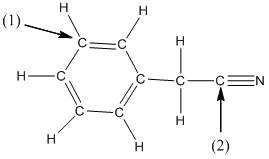
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





