
Principles Of Chemistry: A Molecular Approach, Loose-leaf Edition (4th Edition)
4th Edition
ISBN: 9780134989099
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 87E
Interpretation Introduction
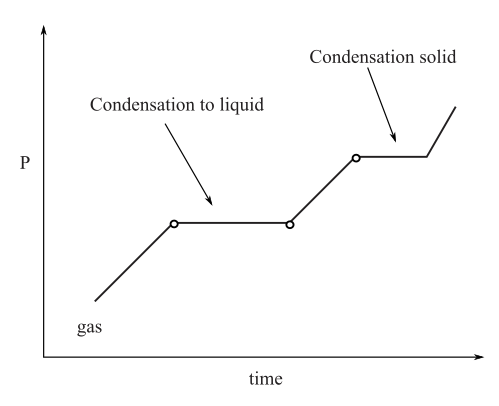
To draw: A graph analogous to the heating curve for water.
Graph analogous to heating curve of water:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Curved arrows were used to generate the significant resonance structure and labeled the most significant contribute. What are the errors in these resonance mechanisms. Draw out the correct resonance mechanisms with an brief explanation.
What are the:
нсе
* Moles of Hice while given: a) 10.0 ml 2.7M ?
6) 10.ome 12M ?
You are asked to use curved arrows to generate the significant resonance structures for the following series of compounds and to label the most significant contributor. Identify the errors that would occur if you do not expand the Lewis structures or double-check the mechanisms. Also provide the correct answers.
Chapter 11 Solutions
Principles Of Chemistry: A Molecular Approach, Loose-leaf Edition (4th Edition)
Ch. 11 - Prob. 1SAQCh. 11 - Q2. Liquid nitrogen boils at 77 K. The image shown...Ch. 11 - Q3. Based on the expected intermolecular forces,...Ch. 11 - Q4. Which substance experiences dipole–dipole...Ch. 11 - Q5. One of these substances is a liquid at room...Ch. 11 - Prob. 6SAQCh. 11 - Q7. Determine the amount of heat (in kJ) required...Ch. 11 - Prob. 8SAQCh. 11 - Prob. 9SAQCh. 11 - Prob. 10SAQ
Ch. 11 - Prob. 1ECh. 11 - 2. Why are intermolecular forces important?
Ch. 11 - 3. What are the main properties of liquids (in...Ch. 11 - 4. What are the main properties of solids (in...Ch. 11 - Prob. 5ECh. 11 - Prob. 6ECh. 11 - Prob. 7ECh. 11 - Prob. 8ECh. 11 - Prob. 9ECh. 11 - Prob. 10ECh. 11 - Prob. 11ECh. 11 - Prob. 12ECh. 11 - 13. What is hydrogen bonding? How can you predict...Ch. 11 - Prob. 14ECh. 11 - Prob. 15ECh. 11 - Prob. 16ECh. 11 - Prob. 17ECh. 11 - Prob. 18ECh. 11 - 19. Why is vaporization endothermic? Why is...Ch. 11 - 20. How is the volatility of a substance related...Ch. 11 - 21. What is the heat of vaporization for a liquid...Ch. 11 - 22. Explain the process of dynamic equilibrium....Ch. 11 - Prob. 23ECh. 11 - Prob. 24ECh. 11 - Prob. 25ECh. 11 - 26. What is the Clausius–Clapeyron equation and...Ch. 11 - Prob. 27ECh. 11 - Prob. 28ECh. 11 - Prob. 29ECh. 11 - Prob. 30ECh. 11 - 31. Examine the heating curve for water in Section...Ch. 11 - Prob. 32ECh. 11 - Prob. 33ECh. 11 - Prob. 34ECh. 11 - Prob. 35ECh. 11 - Prob. 36ECh. 11 - Prob. 37ECh. 11 - Prob. 38ECh. 11 - 39. Arrange these compounds in order of increasing...Ch. 11 - Prob. 40ECh. 11 - Prob. 41ECh. 11 - Prob. 42ECh. 11 - Prob. 43ECh. 11 - Prob. 44ECh. 11 - Prob. 45ECh. 11 - Prob. 46ECh. 11 - Prob. 47ECh. 11 - Prob. 48ECh. 11 - Prob. 49ECh. 11 - 50. Explain why the viscosity of multigrade motor...Ch. 11 - 51. Water in a glass tube that contains grease or...Ch. 11 - Prob. 52ECh. 11 - Prob. 53ECh. 11 - Prob. 54ECh. 11 - Prob. 55ECh. 11 - Prob. 56ECh. 11 - 57. The human body obtains 915 kJ of energy from a...Ch. 11 - Prob. 58ECh. 11 - Prob. 59ECh. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Prob. 62ECh. 11 - Prob. 63ECh. 11 - Prob. 64ECh. 11 - Prob. 65ECh. 11 - Prob. 66ECh. 11 - Prob. 67ECh. 11 - Prob. 68ECh. 11 - Prob. 69ECh. 11 - Prob. 70ECh. 11 - Prob. 71ECh. 11 - 72. How much heat (in kJ) is evolved in converting...Ch. 11 - Prob. 73ECh. 11 - 74. Consider the phase diagram for iodine shown...Ch. 11 - Prob. 75ECh. 11 - Prob. 76ECh. 11 - Prob. 77ECh. 11 - Prob. 78ECh. 11 - Prob. 79ECh. 11 - Prob. 80ECh. 11 - Prob. 81ECh. 11 - 82. How is the density of solid water compared to...Ch. 11 - Prob. 83ECh. 11 - Prob. 84ECh. 11 - Prob. 85ECh. 11 - Prob. 86ECh. 11 - Prob. 87ECh. 11 - Prob. 88ECh. 11 - Prob. 89ECh. 11 - 90. A sample of steam with a mass of 0.552 g and...Ch. 11 - Prob. 91ECh. 11 - Prob. 92ECh. 11 - Prob. 93ECh. 11 - 94. A sealed flask contains 0.55 g of water at 28...Ch. 11 - Prob. 95ECh. 11 - 96. Consider a planet where the pressure of the...Ch. 11 - Prob. 97ECh. 11 - 98. Given that the heat of fusion of water is...Ch. 11 - 99. The heat of combustion of CH4 is 890.4 kJ/mol,...Ch. 11 - Prob. 100ECh. 11 - Prob. 101ECh. 11 - 102. Butane (C4H10) has a heat of vaporization of...Ch. 11 - Prob. 103ECh. 11 - 104. One prediction of global warming is the...Ch. 11 - Prob. 105ECh. 11 - Prob. 106ECh. 11 - Prob. 107ECh. 11 - Prob. 108ECh. 11 - Prob. 109ECh. 11 - Prob. 110ECh. 11 - Prob. 111ECh. 11 - Prob. 112ECh. 11 - Prob. 113QGWCh. 11 - Prob. 114QGWCh. 11 - Prob. 115QGWCh. 11 - Prob. 116QGWCh. 11 - 117. Students in a chemistry laboratory course are...
Knowledge Booster
Similar questions
- how to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forwardAssign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forward
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY