
Basic Chemistry Plus Mastering Chemistry With Pearson Etext -- Access Card Package (6th Edition)
6th Edition
ISBN: 9780134983783
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11, Problem 85UTC
(a)
Interpretation Introduction
Interpretation:
From the given diagram, the gas sample at lowest pressure needs to be determined.
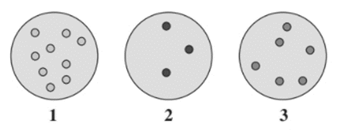
Concept Introduction :
The pressure depends on the number of gaseous molecules in a sample and space between them.
(b)
Interpretation Introduction
Interpretation:
From the given diagram, the gas sample at highest pressure needs to be determined.
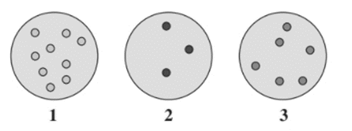
Concept Introduction :
The pressure depends on the number of gaseous molecules in a sample and space between them.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Photochemical smog is formed in part by the action of light on nitrogen dioxide. The wavelength of radiation absorbed by NO2 in this reaction is 197 nm.(a) Draw the Lewis structure of NO2 and sketch its π molecular orbitals.(b) When 1.56 mJ of energy is absorbed by 3.0 L of air at 20 °C and 0.91 atm, all the NO2 molecules in this sample dissociate by the reaction shown. Assume that each absorbed photon leads to the dissociation (into NO and O) of one NO2 molecule. What is the proportion, in parts per million, of NO2 molecules in this sample? Assume that the sample behaves ideally.
Correct each molecule in the drawing area below so that it has the skeletal ("line") structure it would have if it were
dissolved in a 0.1 M aqueous solution of HCI.
If there are no changes to be made, check the No changes box under the drawing area.
No changes.
HO
Explanation
Check
NH,
2
W
O
:□
G
©2025 M
unter Accessibility
An expression for the root mean square velocity, vrms, of a gas was derived. Using Maxwell’s velocity distribution, one can also calculate the mean velocity and the most probable velocity (mp) of a collection of molecules. The equations used for these two quantities are vmean=(8RT/πM)1/2 and vmp=(2RT/M)1/2 These values have a fixed relationship to each other.(a) Arrange these three quantities in order of increasing magnitude.(b) Show that the relative magnitudes are independent of the molar mass of the gas.(c) Use the smallest velocity as a reference for establishing the order of magnitude and determine the relationship between the larger and smaller values.
Chapter 11 Solutions
Basic Chemistry Plus Mastering Chemistry With Pearson Etext -- Access Card Package (6th Edition)
Ch. 11.1 - Prob. 1PPCh. 11.1 - Prob. 2PPCh. 11.1 - Prob. 3PPCh. 11.1 - Prob. 4PPCh. 11.1 - Prob. 5PPCh. 11.1 - Prob. 6PPCh. 11.1 - Prob. 7PPCh. 11.1 - Prob. 8PPCh. 11.2 - Prob. 9PPCh. 11.2 - Prob. 10PP
Ch. 11.2 - Prob. 11PPCh. 11.2 - Prob. 12PPCh. 11.2 - Prob. 13PPCh. 11.2 - Prob. 14PPCh. 11.2 - Prob. 15PPCh. 11.2 - Prob. 16PPCh. 11.2 - Prob. 17PPCh. 11.2 - Prob. 18PPCh. 11.2 - Prob. 19PPCh. 11.2 - Prob. 20PPCh. 11.2 - Prob. 21PPCh. 11.2 - Prob. 22PPCh. 11.2 - Prob. 23PPCh. 11.2 - Prob. 24PPCh. 11.3 - Prob. 25PPCh. 11.3 - Prob. 26PPCh. 11.3 - Prob. 27PPCh. 11.3 - Prob. 28PPCh. 11.3 - Prob. 29PPCh. 11.3 - Prob. 30PPCh. 11.3 - Prob. 31PPCh. 11.3 - Prob. 32PPCh. 11.4 - Prob. 33PPCh. 11.4 - Prob. 34PPCh. 11.4 - Prob. 35PPCh. 11.4 - Prob. 36PPCh. 11.4 - Prob. 37PPCh. 11.4 - Prob. 38PPCh. 11.4 - Prob. 39PPCh. 11.4 - Explain each of the following observations: a....Ch. 11.4 - A tank contains isoflurane, an inhaled anesthetic,...Ch. 11.4 - Prob. 42PPCh. 11.5 - Prob. 43PPCh. 11.5 - Prob. 44PPCh. 11.5 - Prob. 45PPCh. 11.5 - Prob. 46PPCh. 11.5 - Prob. 47PPCh. 11.5 - Prob. 48PPCh. 11.6 - Prob. 49PPCh. 11.6 - Prob. 50PPCh. 11.6 - Prob. 51PPCh. 11.6 - Prob. 52PPCh. 11.6 - Prob. 53PPCh. 11.6 - Prob. 54PPCh. 11.7 - Prob. 55PPCh. 11.7 - What is the volume, in liters, of 4.00 mol of...Ch. 11.7 - An oxygen gas container has a volume of 20.0 L....Ch. 11.7 - Prob. 58PPCh. 11.7 - A 25.0-g sample of nitrogen, N2 , has a volume of...Ch. 11.7 - A 0.226-g sample of carbon dioxide, CO2 , has a...Ch. 11.7 - Prob. 61PPCh. 11.7 - Prob. 62PPCh. 11.7 - Prob. 63PPCh. 11.7 - Prob. 64PPCh. 11.8 - HCl reacts with magnesium metal to produce...Ch. 11.8 - When heated to 350Cat0.950atm , ammonium nitrate...Ch. 11.8 - Butane undergoes combustion when it reacts with...Ch. 11.8 - Potassium nitrate decomposes to potassium nitrite...Ch. 11.8 - Prob. 69PPCh. 11.8 - Nitrogen dioxide reacts with water to produce...Ch. 11.9 - Prob. 71PPCh. 11.9 - Prob. 72PPCh. 11.9 - Prob. 73PPCh. 11.9 - Prob. 74PPCh. 11.9 - Prob. 75PPCh. 11.9 - Prob. 76PPCh. 11.9 - An air sample in the lungs contains oxygen at 93...Ch. 11.9 - Prob. 78PPCh. 11.9 - Prob. 79PPCh. 11.9 - Prob. 80PPCh. 11.9 - Prob. 81PPCh. 11.9 - Prob. 82PPCh. 11 - Prob. 83UTCCh. 11 - Prob. 84UTCCh. 11 - Prob. 85UTCCh. 11 - Indicate which diagram (1, 2, or 3) represents the...Ch. 11 - A balloon is filled with helium gas with a partial...Ch. 11 - Prob. 88UTCCh. 11 - Prob. 89APPCh. 11 - In the fermentation of glucose (wine making), 780...Ch. 11 - Prob. 91APPCh. 11 - Prob. 92APPCh. 11 - In 1783, Jacques Charles launched his first...Ch. 11 - Prob. 94APPCh. 11 - Prob. 95APPCh. 11 - Prob. 96APPCh. 11 - Prob. 97APPCh. 11 - A steel cylinder with a volume of 15.0 L is filled...Ch. 11 - A sample of gas with a mass of 1.62 g occupies a...Ch. 11 - Prob. 100APPCh. 11 - How many grams of...Ch. 11 - A container is filled with...Ch. 11 - How many liters of H2 gas can be produced at...Ch. 11 - Prob. 104APPCh. 11 - Prob. 105APPCh. 11 - Hydrogen gas can be produced in the laboratory...Ch. 11 - Prob. 107APPCh. 11 - Prob. 108APPCh. 11 - A gas mixture contains oxygen and argon at partial...Ch. 11 - Prob. 110APPCh. 11 - Prob. 111CPCh. 11 - When heated, KClO3 forms KCl and O2 . When a...Ch. 11 - A sample of gas with a mass of 1.020 g occupies a...Ch. 11 - A sample of an unknown gas with a mass of 3.24 g...Ch. 11 - Prob. 115CPCh. 11 - When sensors in a car detect a collision, they...Ch. 11 - Prob. 117CPCh. 11 - Prob. 118CPCh. 11 - Prob. 119CPCh. 11 - A hyperbaric chamber has a volume of 1510 L. How...Ch. 11 - Laparoscopic surgery involves inflating the...Ch. 11 - Prob. 122CPCh. 11 - Prob. 123CPCh. 11 - Prob. 124CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction of solid dimethylhydrazine, (CH3)2N2H2, and liquefied dinitrogen tetroxide, N2O4, has been investigated for use as rocket fuel. The reaction produces the gases carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O), which are ejected in the exhaust gases. In a controlled experiment, solid dimethylhydrazine was reacted with excess dinitrogen tetroxide, and the gases were collected in a closed balloon until a pressure of 2.50 atm and a temperature of 400.0 K were reached.(a) What are the partial pressures of CO2, N2, and H2O?(b) When the CO2 is removed by chemical reaction, what are the partial pressures of the remaining gases?arrow_forwardOne liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forward
- Draw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forwardDraw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward
- 19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forwardIndicate the product of the reaction OH OH CH3-CC- Ph + H2SO4 a 20°C | CH3 Pharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY