
Conceptual Integrated Science
3rd Edition
ISBN: 9780135197394
Author: Hewitt, Paul G., LYONS, Suzanne, (science Teacher), Suchocki, John, Yeh, Jennifer (jennifer Jean)
Publisher: PEARSON EDUCATION (COLLEGE)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 67TE
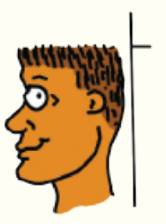
Each night you measure your height just before going to bed. When you arise each morning, you measure your height again and consistently find that you are
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?
For each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank you
Chapter 11 Solutions
Conceptual Integrated Science
Ch. 11 - Prob. 1RCCCh. 11 - Prob. 2RCCCh. 11 - Prob. 3RCCCh. 11 - Prob. 4RCCCh. 11 - Prob. 5RCCCh. 11 - How are the particles in a solid arranged...Ch. 11 - Which occupies the greatest volume: 1 gram of ice,...Ch. 11 - What is it called when evaporation takes place...Ch. 11 - How is sublimation different from evaporation?Ch. 11 - Prob. 10RCC
Ch. 11 - How much heat is needed to melt 1 gram of ice?...Ch. 11 - What happens to the chemical identity of a...Ch. 11 - What is a physical property? A chemical property?Ch. 11 - What is a chemical bond?Ch. 11 - What changes during a chemical reaction?Ch. 11 - Why is the freezing of water considered to be a...Ch. 11 - Prob. 17RCCCh. 11 - Why is the rusting of iron considered to be a...Ch. 11 - Prob. 19RCCCh. 11 - What is the difference between an element and a...Ch. 11 - How many atoms are in one molecule of H3PO4?Ch. 11 - How many atoms of each element are in one molecule...Ch. 11 - What does the chemical formula of a substance tell...Ch. 11 - Prob. 24RCCCh. 11 - Prob. 25RCCCh. 11 - Prob. 26RCCCh. 11 - What is the chemical formula for the compound...Ch. 11 - Why are common names often used for chemical...Ch. 11 - How soon will nanotechnology give rise to...Ch. 11 - Prob. 30TISCh. 11 - Who is the ultimate expert at nanotechnology?Ch. 11 - Prob. 38TCCh. 11 - Rank these substances in order of increasing...Ch. 11 - Rank the following physical and chemical changes...Ch. 11 - Rank these compounds in order of increasing number...Ch. 11 - How has chemistry influenced our modern...Ch. 11 - While visiting a foreign country, a foreign...Ch. 11 - If someone is able to explain an idea to you using...Ch. 11 - What is the best way to really prove to yourself...Ch. 11 - Prob. 46TECh. 11 - Prob. 47TECh. 11 - What is found between two adjacent molecules of a...Ch. 11 - You combine 50mL of water with 50mL of purified...Ch. 11 - Prob. 50TECh. 11 - Which has stronger attractions among its...Ch. 11 - Prob. 52TECh. 11 - Is it possible for air to be in liquid phase?...Ch. 11 - Prob. 54TECh. 11 - The left most diagram below shows the moving...Ch. 11 - The leftmost diagram here shows two phases of a...Ch. 11 - A cotton ball is dipped in alcohol and wiped...Ch. 11 - A skillet is lined with a thin layer of cooking...Ch. 11 - A cotton ball is dipped in alcohol is wiped across...Ch. 11 - Use exercise 58 as an analogy to describe what...Ch. 11 - Prob. 61TECh. 11 - Prob. 62TECh. 11 - Prob. 63TECh. 11 - Why are physical changes typically easier to...Ch. 11 - Prob. 65TECh. 11 - Prob. 66TECh. 11 - Each night you measure your height just before...Ch. 11 - State whether each of the following is an example...Ch. 11 - State whether each of the following is an example...Ch. 11 - How is sugar dissolving in water an example of a...Ch. 11 - Why is the air over a campfire always moist?Ch. 11 - Prob. 72TECh. 11 - Prob. 73TECh. 11 - Each sphere in the diagrams shown here represents...Ch. 11 - Is aging primarily an example of a physical or a...Ch. 11 - Is nuclear fusion, as described in Chapter 10, an...Ch. 11 - Prob. 77TECh. 11 - Prob. 78TECh. 11 - Oxygen atoms are used to make water molecules....Ch. 11 - Oxygen, O2, is certainly good for you. Does it...Ch. 11 - Prob. 81TECh. 11 - Prob. 82TECh. 11 - Which of the following boxes contains only an...Ch. 11 - Prob. 84TECh. 11 - Prob. 85TECh. 11 - What is the chemical name for a compound with the...Ch. 11 - Prob. 87TECh. 11 - Prob. 88TECh. 11 - Is nanotechnology the result of basic or applied...Ch. 11 - How does a scanning probe microscope differ from...Ch. 11 - People often behave differently in a group...Ch. 11 - Prob. 92TECh. 11 - Medicines, such as pain relievers and...Ch. 11 - Your friend smells cinnamon coming from an...Ch. 11 - The British diplomat, physicist, and...Ch. 11 - Prob. 96TDICh. 11 - A calculator is useful but certainly not exciting....Ch. 11 - How might speculations about potential dangers of...Ch. 11 - Over the past 20 years, the average life...Ch. 11 - Prob. 100TDICh. 11 - Prob. 1RATCh. 11 - The molecules in a small collection of molecules...Ch. 11 - The phase in which atoms and molecules no longer...Ch. 11 - Prob. 4RATCh. 11 - Prob. 5RATCh. 11 - Prob. 6RATCh. 11 - Which is an example of a chemical change? a Water...Ch. 11 - If you burn 50kg of wood and produce 10g of ash,...Ch. 11 - If you have one molecule of TiO2, how many...Ch. 11 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
Given the end results of the two types of division, why is it necessary for homologs to pair during meiosis and...
Concepts of Genetics (12th Edition)
Distinguish between microevolution, speciation, and macroevolution.
Campbell Essential Biology (7th Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Look at the relative positions of each pair of atoms listed here in the periodic table. How many core electrons...
Organic Chemistry (8th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forwardWhat are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forward
- A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forward
- Describe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forwardDiscuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward
- 3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forwardA 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College


An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY