
Why do ethanol and dimethyl ether have such different properties even though they have same chemical formula?
To determine: Why do ethanol and dimethyl ether have such different properties even when they have same chemical formula?
Answer to Problem 1E
Solution:
The difference is caused because of different functional group present in both the compounds. Ethanol contains alcohol (-OH) functional group while dimethyl ether contains ether (-O-) group.
Explanation of Solution
The physical and chemical properties of any compound is closely related with its structure and bonding pattern.
So, to understand the difference between properties of the given compounds their structural formula are very important to be discussed.
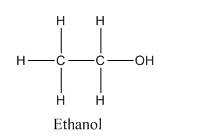
Chemical formula of Ethanol : C2H6O or , C2H5OH
Structural formula of Ethanol :
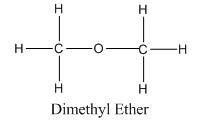
Chemical formula of Dimethyl ether : C2H6O or , CH3-O-CH3
Structural formula of Ethanol :
The difference can be easily seen, that in one hand, where ethanol is a polar molecule with terminal −OH group having electronegativity difference and the ethanol molecules are attached together by hydrogen bonding as the H-atom is directly attached to electronegative O-atom.
While in other hand, dimethyl ether has two methyl groups attached to one other by (-O-) group on either sides which generates a regular dipole-dipole interactions.
The two types of bonding pattern bring changes in the properties of the two compounds.
Also, there are many more functional aspects of both the functional groups which change the properties of compounds instead of having same chemical formula.
The difference in functional groups of ethanol and dimethyl ether brings the difference in their properties.
Want to see more full solutions like this?
Chapter 11 Solutions
Solutions Manual For Chemistry: Structure And Properties
- In general, which is more polar, the stationary phase or the mobile phase? The stationary phase is always more polar The mobile phase is always more polar It depends on our choices for both stationary and mobile phase Their polarity doesn't really matter so we never consider itarrow_forwardPlease helparrow_forwardDraw the mechanism of aspirin synthesis in an basic medium and in a neutral medium, showing the attacks and the process for the formation of the product.arrow_forward
- Na :S f. F NO2arrow_forwardQ1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. + CI OH woཡི།༠w Br H مه D CI ပ။ Br H, Br Br H₂N OMe R IN Ill N S H CI Br CI CI D OH H 1/111arrow_forwardDraw the two products of the reaction. H₂C. CH₂ H :0: CH3 CH₂ +1arrow_forward
- 93 = Volume 93 = 5.32× 10 3 -23 ст a √ 1073 5.32× 10 3 cm³arrow_forwardASP.....arrow_forwardQuestion 7 (10 points) Identify the carboxylic acid present in each of the following items and draw their structures: Food Vinegar Oranges Yogurt Sour Milk Pickles Acid Structure Paragraph ✓ BI UAE 0118 + v Task: 1. Identify the carboxylic acid 2. Provide Name 3. Draw structure 4. Take a picture of your table and insert Add a File Record Audio Record Video 11.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning



