
EBK STUDY GUIDE TO ACCOMPANY CHEMISTRY:
7th Edition
ISBN: 9781119360889
Author: HYSLOP
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 139RQ
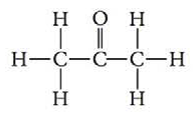
Should acetone molecules be attracted to water molecules more strongly than to other acetone molecules? Explain your answer. The structure of acetone is as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
draw and label the maximum number of water molecules that could hydrogen bond to one central water molecule.
A change in color of a hydrate salt after heating indicates the removal of water molecules. true or false?
Which of the following is FALSE about the water molecule?
- Intermolecular forces among water molecules are the least extensive when it is in liquid form.
- The atoms comprising the water molecule are covalently bonded with one another.
- Its molecular geometry is not linear.
- The H atoms bear the partial positive charge.
What force(s) is(are) responsible for the higher heat of vaporization of acetone compared to benzene?
Chapter 11 Solutions
EBK STUDY GUIDE TO ACCOMPANY CHEMISTRY:
Ch. 11 - Prob. 1PECh. 11 - List the following in order of their boiling...Ch. 11 - Propylamine and trimethylamine have the same...Ch. 11 - People living in arid, dry, regions can cool their...Ch. 11 - Use the kinetic molecular theory to explain why...Ch. 11 - Considering Figure 11.24, in which direction...Ch. 11 - Suppose a liquid is in equilibrium with its vapor...Ch. 11 - The Dead Sea is approximately 1300 ft below sea...Ch. 11 - The atmospheric pressure at the summit of Mt....Ch. 11 - Benzene has a boiling point of 80.1C, and a...
Ch. 11 - Steam can cause more severe bums than water, even...Ch. 11 - The equilibrium line from point B to D in Figure...Ch. 11 - What phase changes will occur if water at 20C and...Ch. 11 - Prob. 14PECh. 11 - Use Le Chtelier's principle to predict how a...Ch. 11 - Prob. 16PECh. 11 - At 0.00C, hexane, C6H14, has a vapor pressure of...Ch. 11 - Prob. 18PECh. 11 - Chromium crystallizes in a body-centered cubic...Ch. 11 - What is the ratio of the ions in the unit cell of...Ch. 11 - Polonium is the only metal known to crystallize in...Ch. 11 - Use the data in the previous Practice Exercise to...Ch. 11 - Stearic acid is an organic acid that has a chain...Ch. 11 - Boron nitride, which has the empirical formula BN,...Ch. 11 - Crystals of elemental sulfur are easily crushed...Ch. 11 - 11.1 Why are the intermolecular attractive forces...Ch. 11 - Compare the behavior of gases, liquids, and solids...Ch. 11 - Prob. 3RQCh. 11 - Why do intermolecular attractions weaken as the...Ch. 11 - Prob. 5RQCh. 11 - Define polarizability. How does this property...Ch. 11 - Prob. 7RQCh. 11 - 11.8 Which nonmetals, besides hydrogen, are...Ch. 11 - Prob. 9RQCh. 11 - Which would give a stronger iondipole interaction...Ch. 11 - Prob. 11RQCh. 11 - Prob. 12RQCh. 11 - Intermolecular Forces and Physical...Ch. 11 - Prob. 14RQCh. 11 - Intermolecular Forces and Physical Properties Name...Ch. 11 - Prob. 16RQCh. 11 - Prob. 17RQCh. 11 - Prob. 18RQCh. 11 - Prob. 19RQCh. 11 - Prob. 20RQCh. 11 - Intermolecular Forces and Physical...Ch. 11 - Prob. 22RQCh. 11 - Prob. 23RQCh. 11 - Prob. 24RQCh. 11 - Prob. 25RQCh. 11 - Prob. 26RQCh. 11 - Prob. 27RQCh. 11 - Prob. 28RQCh. 11 - Prob. 29RQCh. 11 - Changes of State and Dynamic Equilibrium What...Ch. 11 - Prob. 31RQCh. 11 - Changes of State and Dynamic Equilibrium
11.32 Why...Ch. 11 - Changes of State and Dynamic Equilibrium
11.33...Ch. 11 - Changes of State and Dynamic Equilibrium
11.34....Ch. 11 - Prob. 35RQCh. 11 - Prob. 36RQCh. 11 - Vapor Pressures of Liquids and Solids
11.37...Ch. 11 - Prob. 38RQCh. 11 - Vapor Pressures of Liquids and Solids 11.39 What...Ch. 11 - Vapor Pressures of Liquids and Solids Why does...Ch. 11 - Vapor Pressures of Liquids and Solids Why do we...Ch. 11 - Prob. 42RQCh. 11 - Boiling Points of Liquids Why does the boiling...Ch. 11 - Boiling Points of Liquids Mt. Kilimanjaro in...Ch. 11 - Boiling Points of Liquids
11.45. When liquid...Ch. 11 - Prob. 46RQCh. 11 - Boiling Points of Liquids Butane, C4H10, has a...Ch. 11 - Boiling Points of Liquids
11.48. Why does have a...Ch. 11 - Boiling Points of Liquids An HF bond is more polar...Ch. 11 - Energy and Changes of State The following is a...Ch. 11 - Energy and Changes of State
11.51 Why is larger...Ch. 11 - Energy and Changes of State Would the heat of...Ch. 11 - Energy and Changes of State Hurricanes can travel...Ch. 11 - Energy and Changes of State Ethanol (grain...Ch. 11 - Energy and Changes of State A burn caused by steam...Ch. 11 - Energy and Changes of State
11.56 Arrange the...Ch. 11 - Prob. 57RQCh. 11 - Phase Diagrams
11.58 Define critical temperature...Ch. 11 - Phase Diagrams What is a supercritical fluid? Why...Ch. 11 - Phase Diagrams
11.60 What phases of a substance...Ch. 11 - Prob. 61RQCh. 11 - Prob. 62RQCh. 11 - Phase Diagrams Sketch a generic phase diagram that...Ch. 11 - Phase Diagrams
11.64 What is the significance of...Ch. 11 - Prob. 65RQCh. 11 - Le Chtelier's Principle and Changes of State State...Ch. 11 - Le Châtelier's Principle and Changes of...Ch. 11 - Le Chtelier's Principle and Changes of State Use...Ch. 11 - Le Chtelier's Principle and Changes of State Use...Ch. 11 - Le Châtelier's Principle and Changes of...Ch. 11 - Determining Heats of Vaporization According to the...Ch. 11 - Determining Heats of Vaporization Why can't...Ch. 11 - Determining Heats of Vaporization Why can any...Ch. 11 - Prob. 74RQCh. 11 - Prob. 75RQCh. 11 - Prob. 76RQCh. 11 - Determining the Structure of Solids What...Ch. 11 - Determining the Structure of Solids
11.78 The...Ch. 11 - The figure below illustrates the way the atoms of...Ch. 11 - Make a sketch of a layer of sodium ions and...Ch. 11 - 11.81 How do the crystal structures of copper and...Ch. 11 - Determining the Structure of Solids
11.82 What...Ch. 11 - Determining the Structure of Solids Only 14...Ch. 11 - Determining the Structure of Solids Write the...Ch. 11 - Determining the Structure of Solids Why cant...Ch. 11 - Prob. 86RQCh. 11 - Crystal Types and Physical Properties
11.87 What...Ch. 11 - Prob. 88RQCh. 11 - Prob. 89RQCh. 11 - Prob. 90RQCh. 11 - Intermolecular Forces and Physical Properties What...Ch. 11 - Intermolecular Forces and Physical Properties What...Ch. 11 - Intermolecular Forces and Physical Properties...Ch. 11 - Prob. 94RQCh. 11 - 11.95 Consider the compounds (chloroform, an...Ch. 11 - 11.96 Carbon dioxide does not liquefy at...Ch. 11 - Prob. 97RQCh. 11 - Prob. 98RQCh. 11 - Prob. 99RQCh. 11 - Prob. 100RQCh. 11 - 11.101 The following are the vapor pressures of...Ch. 11 - 11.102 The boiling points of some common...Ch. 11 - 11.103 Using the information in Problem 11.101,...Ch. 11 - 11.104 Using the information in Problem 11.102,...Ch. 11 - 11.105 What intermolecular forces must the...Ch. 11 - 11.106 What intermolecular attractions will be...Ch. 11 - Energy and Changes of State The molar heat of...Ch. 11 - Energy and Changes of State The molar heat of...Ch. 11 - *11.109 Suppose 45.0 g of water at is added to...Ch. 11 - A cube of solid benzene (C6H6) at its melting...Ch. 11 - Prob. 111RQCh. 11 - Prob. 112RQCh. 11 - Prob. 113RQCh. 11 - Prob. 114RQCh. 11 - Prob. 115RQCh. 11 - Prob. 116RQCh. 11 - Determining Heats of Vaporization
*11.117 Mercury...Ch. 11 - Prob. 118RQCh. 11 - Prob. 119RQCh. 11 - *11.120 If the vapor pressure of ethylene glycol...Ch. 11 - Determining the Structure of Solids
11.121 How...Ch. 11 - 11.122 How many copper atoms are within the...Ch. 11 - The atomic radius of nickel is 1.24 . Nickel...Ch. 11 - 11.124 Silver forms face-centered cubic crystals....Ch. 11 - Potassium ions have a radius of 133 pm, and...Ch. 11 - 11.126 The unit cell edge in sodium chloride has a...Ch. 11 - Prob. 127RQCh. 11 - Prob. 128RQCh. 11 - *11.129 Cesium chloride forms a simple cubic...Ch. 11 - 11.130 Rubidium chloride has the rock salt...Ch. 11 - Prob. 131RQCh. 11 - Crystal Types and Physical Properties Elemental...Ch. 11 - Prob. 133RQCh. 11 - Prob. 134RQCh. 11 - Prob. 135RQCh. 11 - Crystal Types and Physical Properties
11.1 36...Ch. 11 - List all of the attractive forces that exist in...Ch. 11 - 11.138 Calculate the mass of water vapor present...Ch. 11 - 11.139 Should acetone molecules be attracted to...Ch. 11 - The following thermochemical equations apply to...Ch. 11 - Melting point is sometimes used as an indication...Ch. 11 - When warm, moist air sweeps in from the ocean and...Ch. 11 - *11.143 Gold crystallizes in a face-centered cubic...Ch. 11 - Gold crystallizes with a face-centered cubic unit...Ch. 11 - Identify the type of unit cell belonging to the...Ch. 11 - Calculate the amount of empty space (in pm3) in...Ch. 11 - Silver has an atomic radius of 144 pm. What would...Ch. 11 - Potassium chloride crystallizes with the rock salt...Ch. 11 - Prob. 149RQCh. 11 - There are 270 Calories in a Hersheys* Milk...Ch. 11 - Prob. 151RQCh. 11 - *11.152 Frecze-drying is a process used to...Ch. 11 - When reporting the vapor pressure for a substance...Ch. 11 - 11.154 Supercritical is used to decaffeinate...Ch. 11 - 11.155 Freshly precipitated crystals are usually...Ch. 11 - 11.156 What are three “everyday” applications of...Ch. 11 - Prob. 157RQCh. 11 - 11.158 Galileo's thermometer is a tube of liquid...Ch. 11 - Use the Clausius-Clapeyron equation to plot the...Ch. 11 - Prob. 160RQCh. 11 - Earlier in this chapter it was noted that the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
16. With care, it’s possible to walk on top of a barrel as it rolls. It is much easier to do this if the barrel...
College Physics: A Strategic Approach (3rd Edition)
1. Which parts of the skeleton belong to the appendicular skeleton? Which belong to the axial skeleton?
Human Anatomy & Physiology (2nd Edition)
There are 25 individuals in population 1, all with genotype AA, and there are 40 individuals in population 2, a...
Campbell Biology (11th Edition)
When working on barley plants, two researchers independently identify a short-plant mutation and develop homozy...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Cooking oil floats on top of water. From this observation, what conclusions can you draw regarding the polarity or hydrogen-bonding ability of molecules found in cooking oil?arrow_forwardTrue or false? Methane (CH4) is more likely In form stronger hydrogen bonding than is water because each methane molecule has twice as many hydrogen alums. Provide a concise explanation of hydrogen bonding to go with your answer.arrow_forward8.52 Rank the following hydrocarbons in order of increasing vapor pressure: C2H6,C10H22,CH4,C7H16,C22H46 .arrow_forward
- Silane SiH4, phosphine (PH3), and hydrogen sulfide (H2S) melt at 185 C, 133 C, and 85 C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?arrow_forwarda) Are there any intermolecular forces (IMF's) between water molecules and cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of AHMIXING be for cyclohexane dissolving in water? b) Are there any IMF's between water molecules and other water molecules? What kind(s)? Given this, what would the magnitude and sign of AHSOLVENT be for cyclohexane dissolving in water? c) Are there any IMF's between cyclohexane molecules and other cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of AHSOLITE be for cyclohexane dissolving in water? d) Why do you think cyclohexane does not dissolve in water? Analyze this the way it was done in class, thinking about the three contributions to AHSOLUTION. What do you think the magnitude and sign of AHSOLUTION would be for dissolving cyclohexane in water? (Explain.) Would you expect these substances to dissolve in each other? Why? 2. a) Are there any IMF's between cyclohexane molecules and vegetable…arrow_forwardCompound y has a heat of fusion of 361 J/g. The solid form has a specific heat capacity of 0.353 J/gºC and the liquid form has a specific heat capacity of 2.31 J/gºC. The freezing point of compound y is -15.6ºC. How much energy will it take to convert a 330.4 g solid sample of y at -33.3ºC to a sample at 43.6ºC? Give your answer in kJ, make sure it is rounded to the correct number of sig figs.arrow_forward
- Define the Hydrogen bonding in water ?arrow_forwardIn a body of water, the surface tension caused by the attraction between water molecules is strong. Which of the following is a consequence of this property? Water is considered to be a universal solvent. When water freezes, the molecules move farther apart. Only a limited amount of solute can be dissolved in a sample of water. Objects with a higher density than water can be observed floating on water.arrow_forwardWhat can you conclude about the strength of the interactions between water particles compared to the strength of the interactions between particles of the alcohol? Use the information from the table of values.arrow_forward
- MCQ 144: The attraction of water molecules to the ions on the surface of ionic solid is termed as A. ion-dipole attractions B. dipole interaction C. dipole reaction D. ion-attractionarrow_forwardWhat are adhesive forces? a)The intermolecular attraction forces that make molecules of one substance stick to surfaces with which they come into contact. b)The forces that make some molecules denser than others. c)The forces that attract positive and negative particles to each other. d)The intermolecular attraction forces that make molecules of one substance repel the surfaces with which they come into contact.arrow_forward4. Draw two water molecules and the ions forming from KOH and identify at least one ion-dipole interaction between one water molecule and the ions. Draw at least one hydrogen bond interaction forming between the two water molecules.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY