
Concept explainers
(a)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the
Answer to Problem 11.77AP
The curved arrow mechanism for the reaction is shown below.
Explanation of Solution
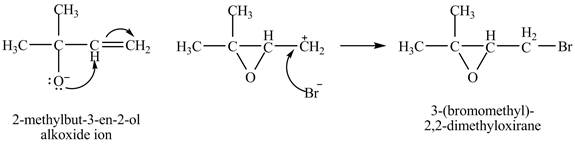
The given reaction is shown below.
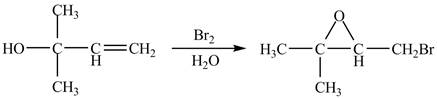
Figure 1
The oxygen atom of the alkoxide ion attack carbon of double bond to form

Figure 2
The curved arrow mechanism for given reaction is shown in Figure 2.
(b)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
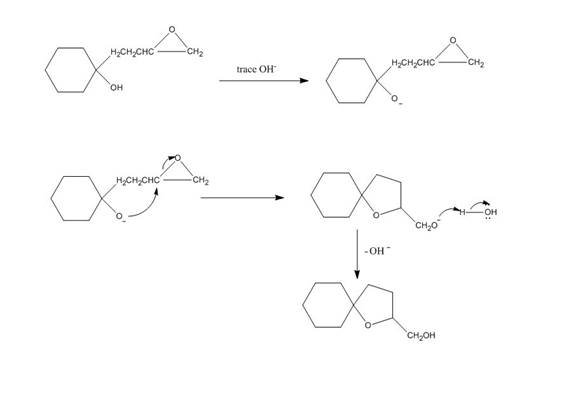
The given reaction is shown below.
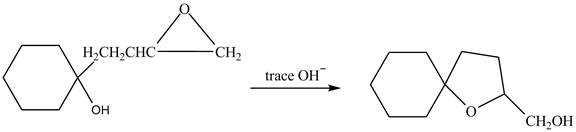
Figure 3
Hydroxide ion abstract the proton from hydroxyl group of given compound. The oxide attacks the carbon of epoxide ring to form a five membered ring. The alkoxide ion formed is protonated in presence of water. The curved arrow mechanism of the given reaction is shown below.
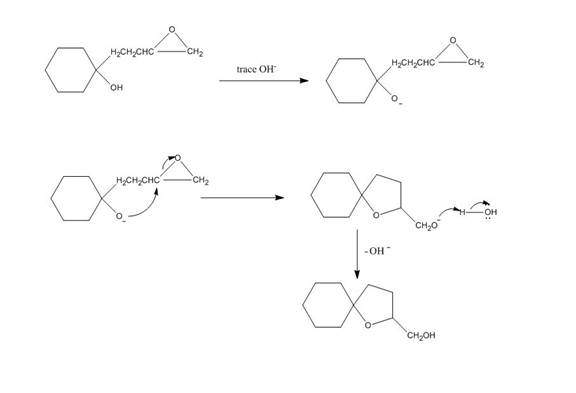
Figure 4
The curved arrow mechanism for given reaction is shown in Figure 4.
(c)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
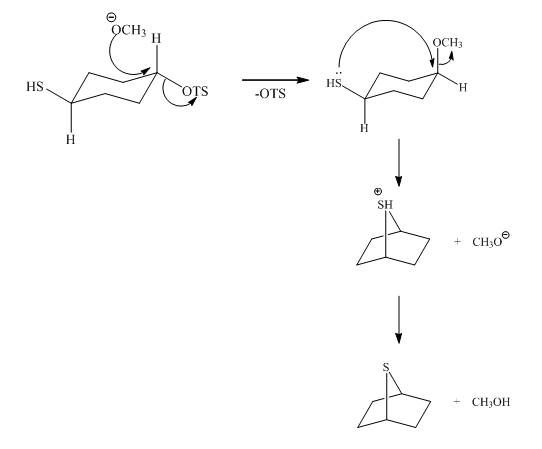
Explanation of Solution
The given reaction is shown below.
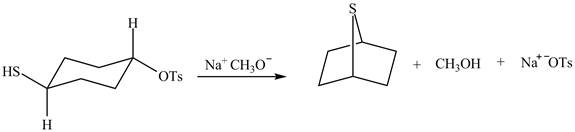
Figure 5
The methoxy group attacks the carbon containing sulfonate ester group. The nucleophilic displacement reaction occurs. The sulfonate ester group is displaced by methoxy group. Then neighbouring group participation of sulfur atom displaces the methoxy group. The sulfonium ion formed undergoes deprotonation to form the product. The curved arrow mechanism of the given reaction is shown below.

Figure 6
The curved arrow mechanism for given reaction is shown in Figure 6.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
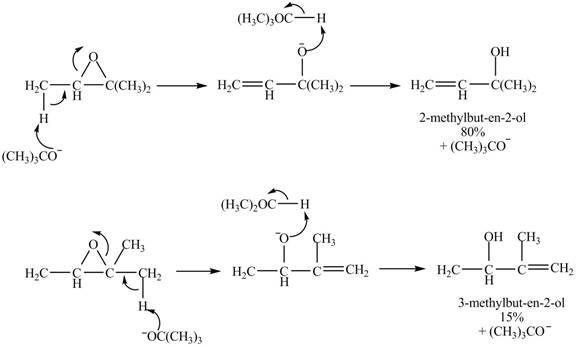
Explanation of Solution
The given reaction is shown below.
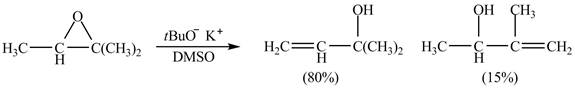
Figure 7
A strong base is used in the reaction; hence elimination reaction will take place. The given compound contains two
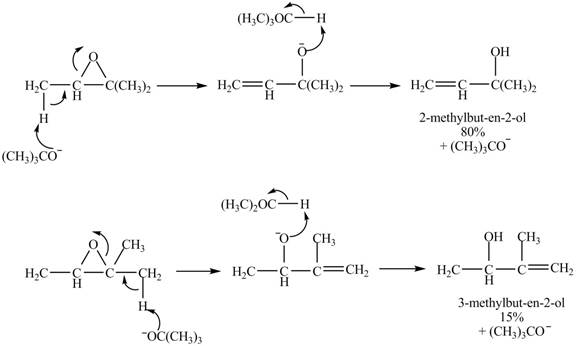
Figure 8
The curved arrow mechanism for given reaction is shown in Figure 8.
(e)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
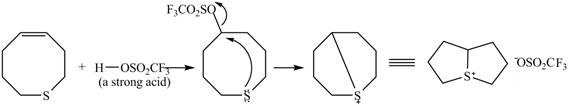
Explanation of Solution
The given reaction is shown below.
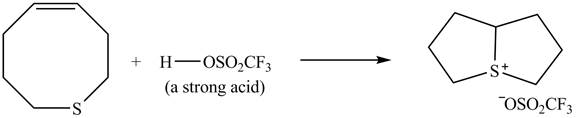
Figure 9
The
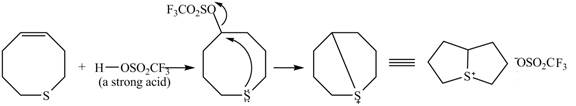
Figure 10
The curved arrow mechanism for given reaction is shown in Figure 10.
(f)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
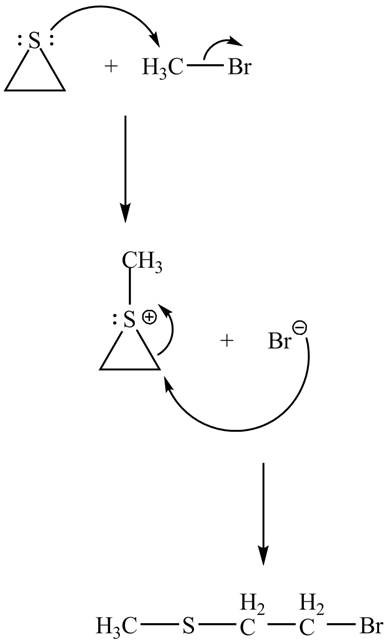
Explanation of Solution
The given reaction is shown below.
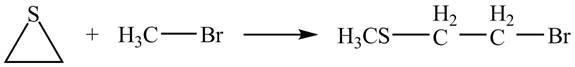
Figure 11
The lone pair of sulphur atom attacks the carbon of methyl bromide. The sulfur atom of the ring gets methylated. Then bromide ion attacks the carbon atom of ring which results in ring opening to form the desired product. The curved arrow mechanism of the given reaction is shown below.

Figure 12
The curved arrow mechanism for given reaction is shown in Figure 12.
(g)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
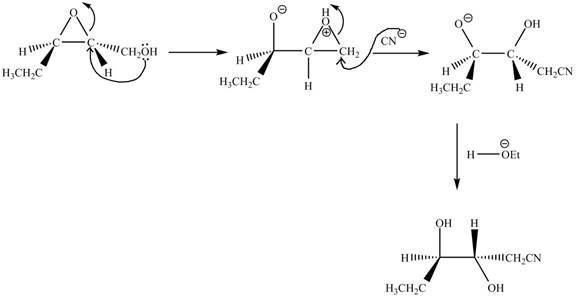
Explanation of Solution
The given reaction is shown below.
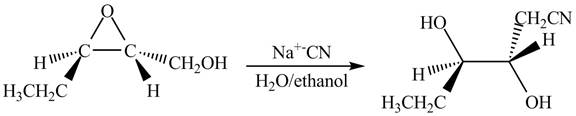
Figure 13
The neighbouring group participation of oxygen atom takes place in the given reaction. The oxygen atom of hydroxyl group attacks the electrophilic carbon atom of the epoxide ring which results in the formation of new epoxide ring. Then cyanide ion attacks at the electrophilic carbon of epoxide which results in ring opening. The oxide ion takes proton form ethanol to form desired product. The curved arrow mechanism for given reaction is shown below.

Figure 14
The curved arrow mechanism for given reaction is shown in Figure 14.
(h)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
Explanation of Solution
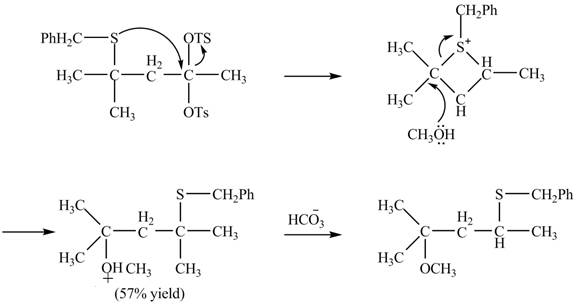
The given reaction is shown below.
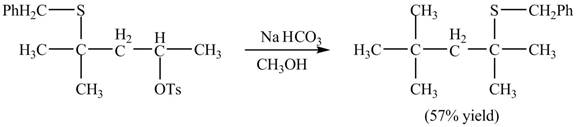
Figure 15
The sulfur atom attacks at the electrophilic carbon atom to form a four membered ring. The nucleophilic attack by methanol at the ring results in ring opening. Then the base abstracts proton from the protonated hydroxyl group to form the desired product. The curved-arrow mechanism for the given reaction is shown below.
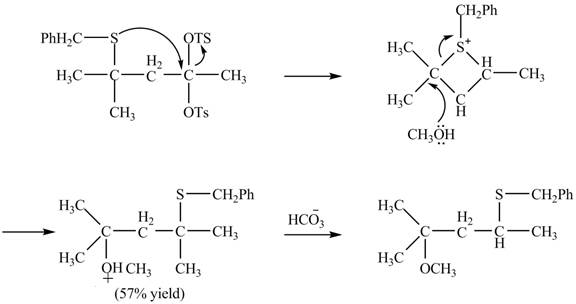
Figure 16
The curved arrow mechanism for given rearrangement is shown in Figure 16.
(i)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
The vicinal
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
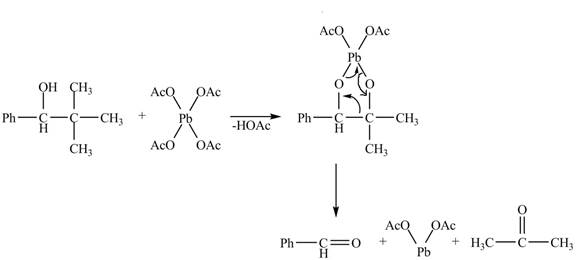
Explanation of Solution
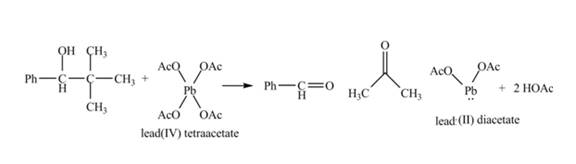
Figure 17
The given compound is a vicinal diol. It will react with the lead tetraacetate to form five membered ring intermediate. The intermediate formed rearranges to form acetone, benzaldehyde and lead diacetate. The curved arrow mechanism for the given reaction is shown below.
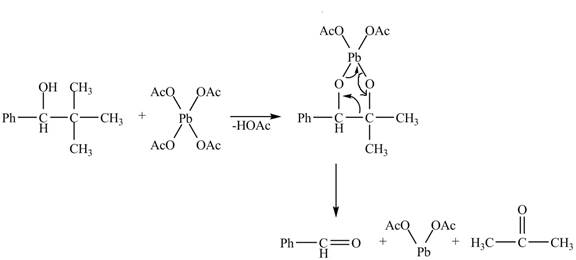
Figure 18
The curved arrow mechanism for given reaction is shown in Figure 18.
Want to see more full solutions like this?
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- dG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forwardThe vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forwardDraw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forward
- From the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forwardPotassium (K) blends with germanium (Ge) to form a Zintl phase with a chemical formula of K4Ge4. Which of the following elements would you expect potassium to blend with to form an alloy? Electronegativities: As (2.0), Cl (3.0), Ge (1.8), K (0.8), S (2.5), Ti (1.5) Group of answer choices Arsenic (As) Sulfur (S) Chlorine (Cl) Titanium (Ti)arrow_forwardConsider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forward
- How many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forwardConsider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forward
- What kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forwardProblem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

