
(a)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The replacement or substitution of one
Answer to Problem 11.60AP
The completed given reaction is shown below.
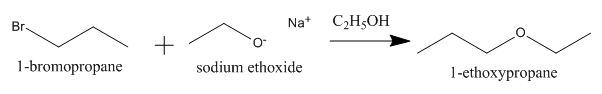
Explanation of Solution
The incomplete given reaction is shown below.
The oxygen atom of sodium ethoxide can act as a nucleophile and attack the carbon atom of

Figure 1
The completed given reaction is shown in Figure 1.
(b)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
Substitution reaction is a type of reaction in which an atom or group of atoms is replaced or substituted by another atom or group of atoms. An elimination reaction is a type of reaction in which two atoms are eliminated in the presence of solvent. Elimination reactions are usually favored in the alcoholic conditions.
Answer to Problem 11.60AP
The completed given reaction is shown below.
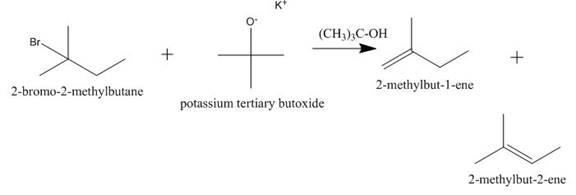
Explanation of Solution
The incomplete given reaction is shown below.
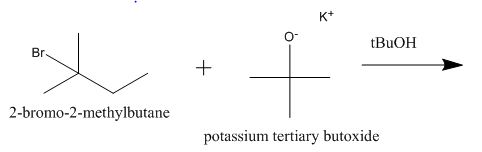
Figure 2
The given

Figure 3
The completed given reaction is shown in Figure 3.
(c)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
Oxymercuration reaction is a type of reaction in which an alkene gets converted to alcohol. The mercuric acetate is used in the reaction as a reagent. This reagent attacks the alkene to form a cyclic intermediate compound which further undergoes reduction to form alcohol.
Answer to Problem 11.60AP
The completed given reaction is shown below.
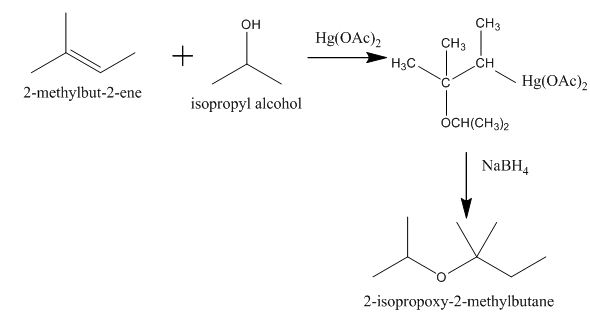
Explanation of Solution
The incomplete given reaction is shown below.
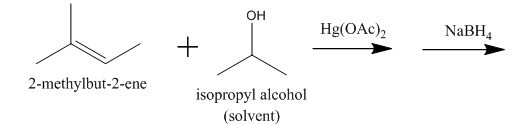
Figure 4
The mercuric acetate
The corresponding chemical reaction is shown below.

Figure 5
The completed given reaction is shown in Figure 5.
(d)
Interpretation:
The given reaction is to be completed with principal organic products. The stereochemistry of the product is to be predicted.
Concept introduction:
An
Answer to Problem 11.60AP
The completed given reaction is shown below.
Explanation of Solution
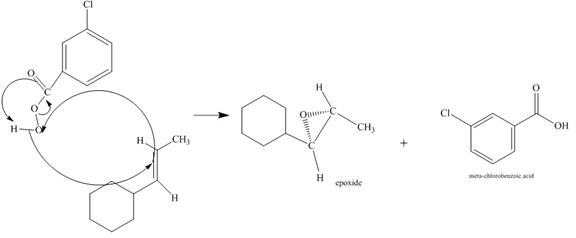
The incomplete given reaction is shown below.
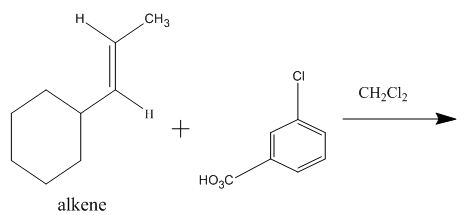
Figure 6
The alkene attacks on the peroxide linkage of the given percarboxylic acid to form an epoixde ring with elimination of carboxylic acid. The oxygen atom of the epoxide will be pointed inwards that is into the plane of the molecule. The corresponding chemical reaction is shown below.
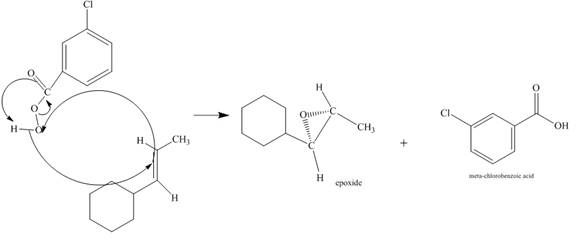
Figure 7
The completed given reaction is shown in Figure 7.
(e)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The addition of one or more than one halogen atom in an alkene is known as halogenation reaction. The pathway of the halogenation reaction depends on the structure of the substrate. In this, the halogen is added to
The compound chromium
Answer to Problem 11.60AP
The completed given reaction is shown below.
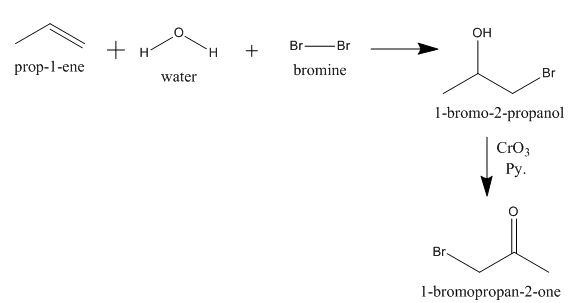
Explanation of Solution
The incomplete given reaction is shown below.
The propene molecule undergoes halohydrin reaction with bromine gas in the presence of water molecule to form bromine substituted alcohol. The alcohol gets oxidized by

Figure 8
The completed given reaction is shown in Figure 8.
(f)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The periodic acid acts as a strong oxidizing agent. The periodic acid reacts with a vicinal diol to form two aldehyde after breaking the carbon-carbon bond between the vicinal diol. The gem diol does not react with periodic acid as vicinal diol.
Answer to Problem 11.60AP
The completed given reaction is shown below.
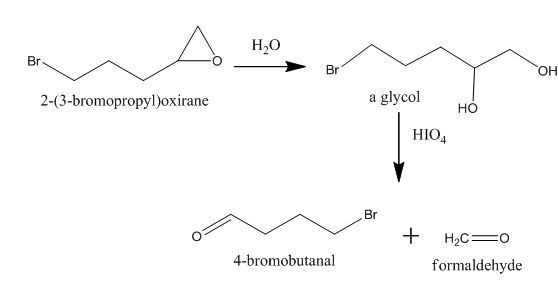
Explanation of Solution
The incomplete given reaction is shown below.
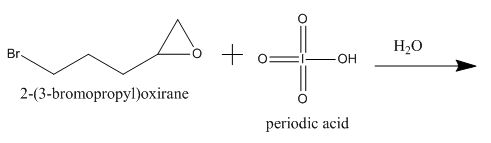
Figure 9
The epoxide is converted into gem diol after the addition of water in it. The gem diol gets converted into two different aldehydes after breaking the bond between carbon atoms attached to the hydroxyl group. The corresponding chemical reaction is shown below.

Figure 10
The completed given reaction is shown in Figure 10.
(g)
Interpretation:
The given reaction is to be completed with principal organic products. The stereochemistry of the product is to be predicted.
Concept introduction:
Potassium permanganate is a strong oxidizing agent. Potassium permanganate can oxidize an alkene into alcohol. It can also oxidize an alcohol into carbonyl compound and the carbonyl compound can be further oxidized to form a carboxylic acid.
Answer to Problem 11.60AP
The completed given reaction is shown below.
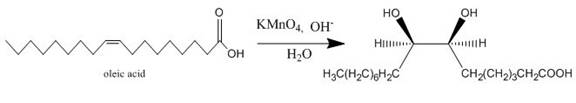
Explanation of Solution
The incomplete given reaction is shown below.
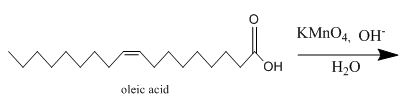
Figure 11
The oleic acid reacts with alkaline
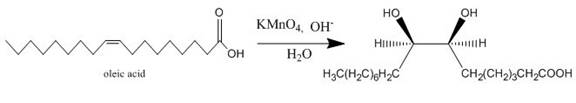
Figure 12
The completed given reaction is shown in Figure 12.
(h)
Interpretation:
The given reaction is to be completed with principal organic products. The stereochemistry of the product is to be predicted.
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are basic or neutral, then the reaction will occur at the less substituted carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
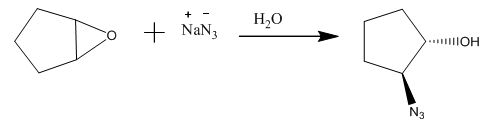
Explanation of Solution
The incomplete given reaction is shown below.
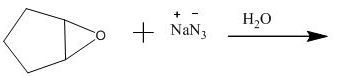
Figure 13
The compound epoxide undergoes ring-opening reaction in the presence of sodium azide. The azide ion acts a nucleophile and attacks on the carbon atom of the epoxide ring to from final compound. The corresponding chemical reaction is shown below.
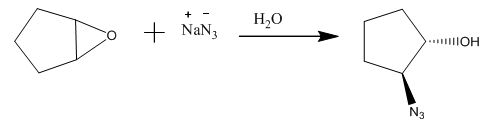
Figure 14
The completed given reaction is shown in Figure 14.
(i)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
Explanation of Solution
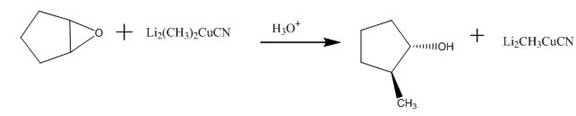
The incomplete given reaction is shown below.
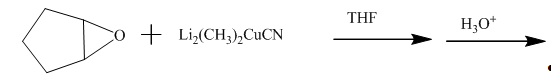
Figure 15
The ether undergoes ring-opening reaction in the presence of acid. The dilithium dimethylcyanocuprate molecule generates a nucleophile

Figure 16
The completed given reaction is shown in Figure 16.
(j)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking the electron-deficient carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
Explanation of Solution
The incomplete given reaction is shown below.
The compound

Figure 17
The completed given reaction is shown in Figure 17.
(k)
Interpretation:
The given reaction is to be completed with principal organic products.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking on the electron-deficient carbon atom.
Answer to Problem 11.60AP
The completed given reaction is shown below.
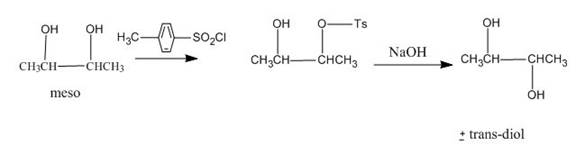
Explanation of Solution
The incomplete given reaction is shown below.
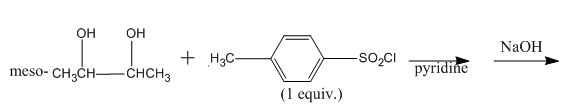
Figure 18
The replacement of the hydroxyl group is not easy. Therefore, alcohol is first converted into tosylate ester with the help of tosyl chloride. The tosylate ester group is then substituted with

Figure 19
The completed given reaction is shown in Figure 19.
Want to see more full solutions like this?
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- 2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forward
- What is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forwardA 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forward
- write the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forwardThe compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forwardCaffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward
- 2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forwardIndicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forwardWrite chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
