
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134421353
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.49APP
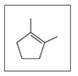
Give the IUPAC name (including cis or trans, if needed) for each of the following: (11.5, 11.6)
a. 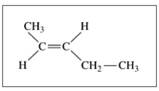 b.
b.
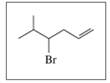
C.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the skeletal structure of the
alkane 4-ethyl-2, 2, 5, 5-
tetramethylnonane. How many
primary, secondary, tertiary, and
quantenary carbons does it have?
Don't used Ai solution
Don't used Ai solution
Chapter 11 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 11.1 - Identify each of the following as a formula of an...Ch. 11.1 - Identify each of the following as a formula of an...Ch. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Prob. 11.5PPCh. 11.1 - Prob. 11.6PPCh. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Draw the condensed structural formula for alkanes...Ch. 11.2 - Draw the condensed structural formula for alkanes...
Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Give the IUPAC name for each of the following a....Ch. 11.3 - Give the TUPAC name for each of the following: a....Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the line-angle formula for each of the...Ch. 11.3 - Prob. 11.18PPCh. 11.4 - Heptane, used as a solvent for rubber cement, has...Ch. 11.4 - Nonane has a density of 0.79 g/mL and boils at 151...Ch. 11.4 - Write the balanced chemical equation for the...Ch. 11.4 - Write the balanced chemical equation for the...Ch. 11.5 - Identify the following as alkanes, alkenes,...Ch. 11.5 - Identify the following as alkanes, alkenes,...Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Draw the condensed structural formula, or...Ch. 11.5 - Prob. 11.28PPCh. 11.6 - Give the IUPAC name for each of the following,...Ch. 11.6 - Give the IUPAC name for each of the following,...Ch. 11.6 - Draw the condensed structural formula for each of...Ch. 11.6 - Prob. 11.32PPCh. 11.7 - Draw the structural formula for the product in...Ch. 11.7 - Draw the structural formula for the product in...Ch. 11.8 - Give the IUPAC name for each of the following: a....Ch. 11.8 - Give the IUPAC name for each of the following: a....Ch. 11.8 - Draw the line-angle formula for each of the...Ch. 11.8 - Draw the line-angle formula for each of the...Ch. 11.8 - Write the balanced chemical equation for the...Ch. 11.8 - Write the balanced chemical equation for the...Ch. 11 - Prob. 11.41UTCCh. 11 - Prob. 11.42UTCCh. 11 - Prob. 11.43UTCCh. 11 - Prob. 11.44UTCCh. 11 - Convert each of the following line-angle formulas...Ch. 11 - Convert each of the following line-angle formulas...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name (including cis or trans, if...Ch. 11 - Give the LUPAC name (including cis or trans, if...Ch. 11 - Prob. 11.51APPCh. 11 - Prob. 11.52APPCh. 11 - Name each of the following aromatic compounds:...Ch. 11 - Prob. 11.54APPCh. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Prob. 11.59APPCh. 11 - Draw the line-angle formula for each of the...Ch. 11 - Write a balanced chemical equation for the...Ch. 11 - Write a balanced chemical equation for the...Ch. 11 - Give the name for the product from the...Ch. 11 - Give the name for the product from the...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Prob. 11.67CPCh. 11 - Prob. 11.68CPCh. 11 - Prob. 11.69CPCh. 11 - Prob. 11.70CPCh. 11 - Prob. 11.71CPCh. 11 - Prob. 11.72CPCh. 11 - Prob. 11.73CPCh. 11 - Margarines are produced from the hydrogenation of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY