
(a)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(a)
Answer to Problem 11.41P
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
Explanation of Solution
The Lewis structure of
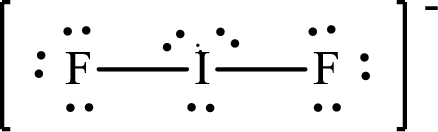
Iodine forms two single bonds with two fluorine atoms and three lone pairs are present on it so the hybridization of iodine in
The
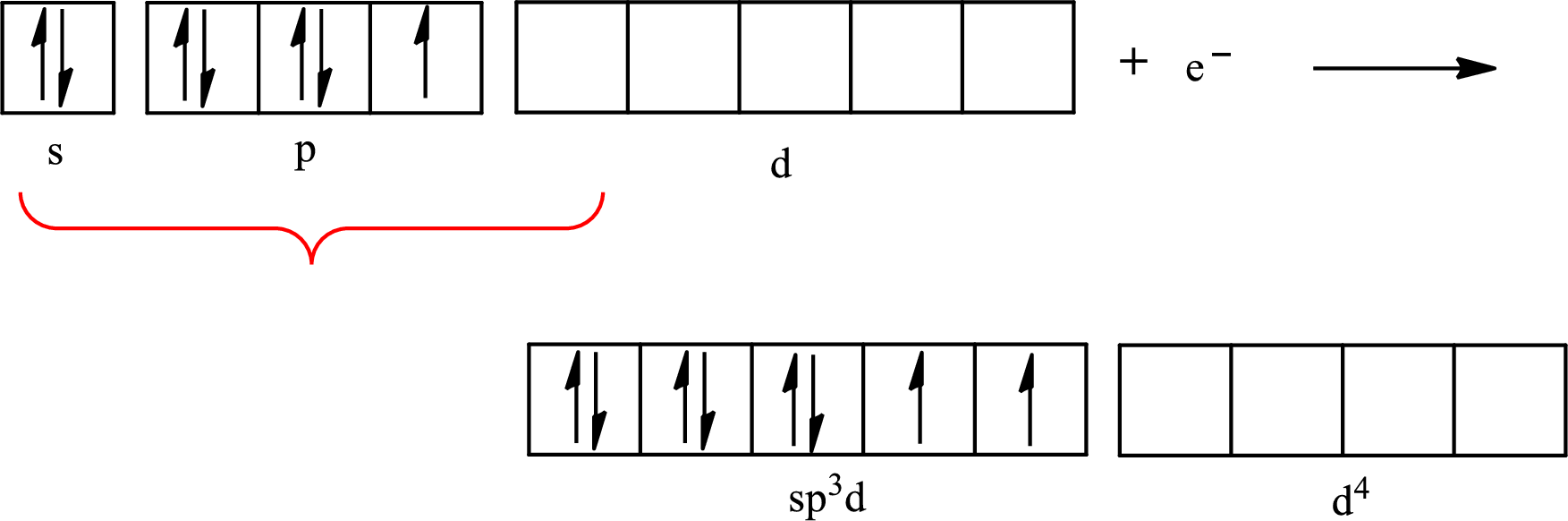
The partial orbital diagram for an isolated
The partial orbital for hybridized
One s orbital, three p orbitals and one d orbital of central atom iodine combine to form five
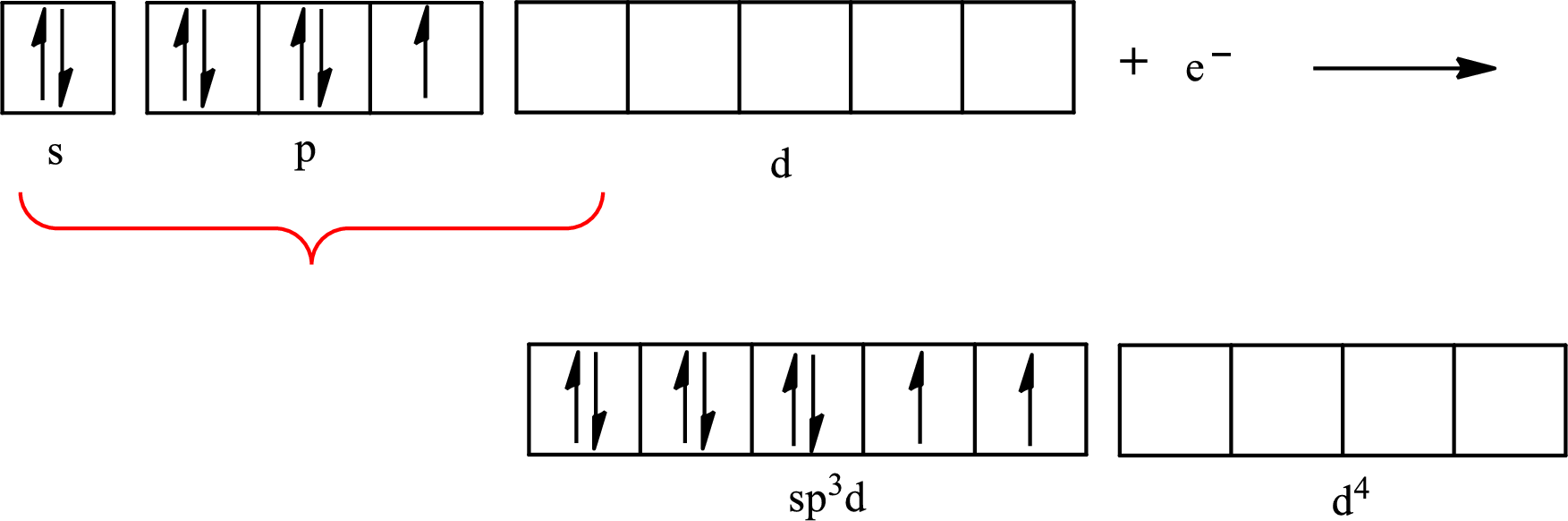
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
(b)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(b)
Answer to Problem 11.41P
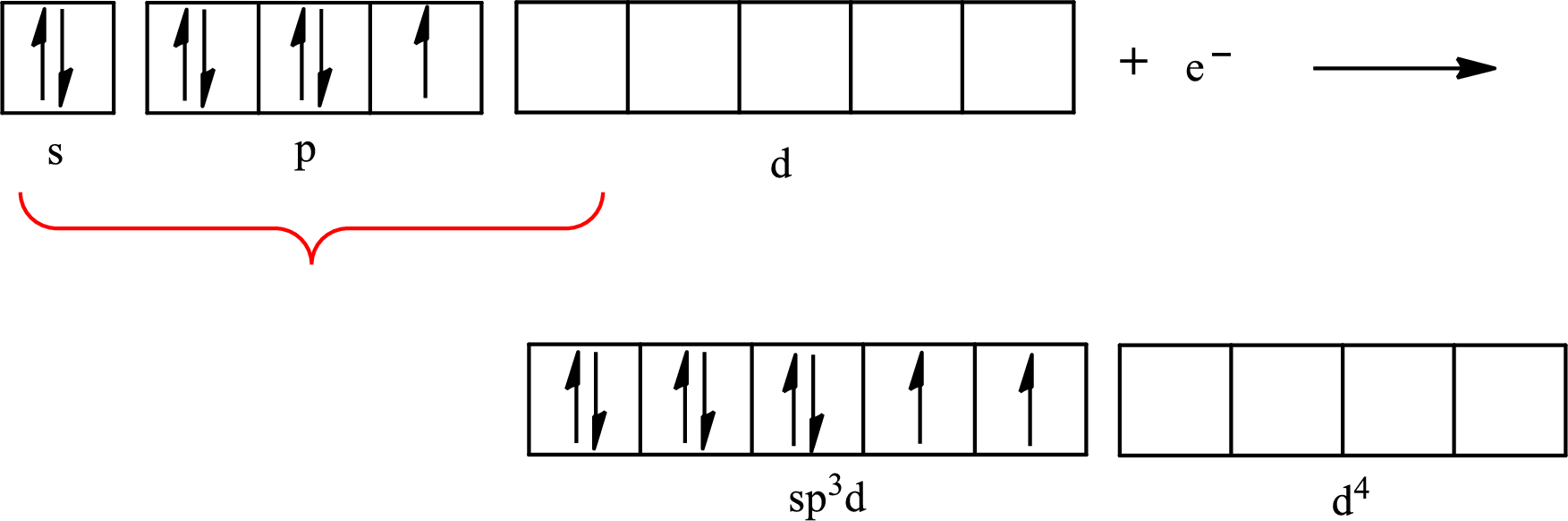
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
Explanation of Solution
The Lewis structure of
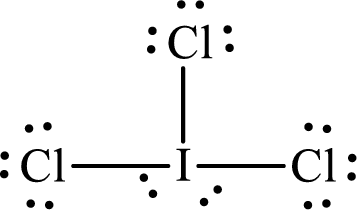
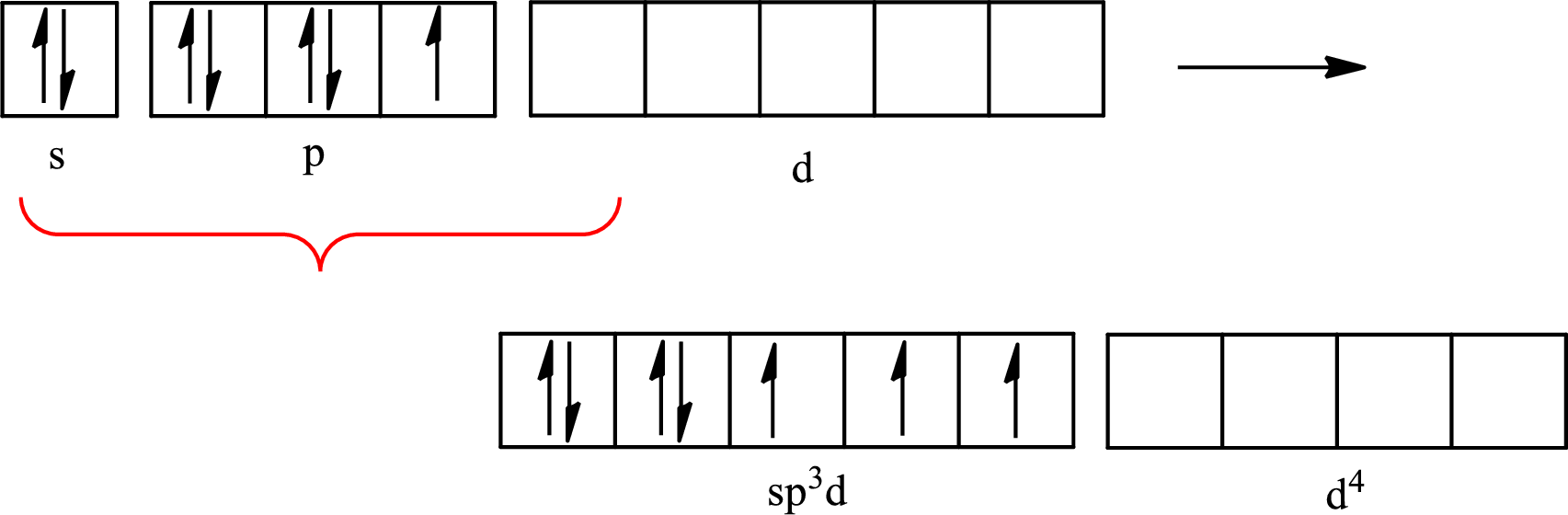
Iodine forms three single bonds with three chlorine atoms and two lone pairs are present on it so five hybrid orbitals are required. The hybridization of
The atomic number of iodine is 53 so its electronic configuration is
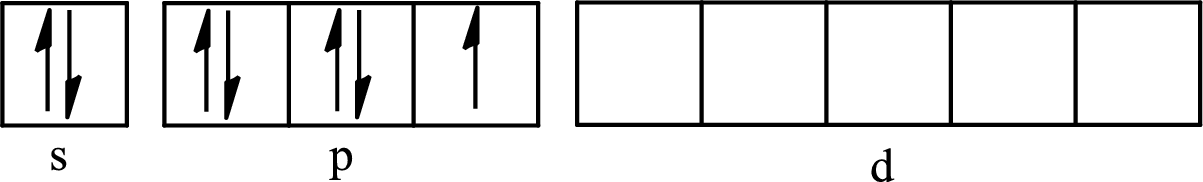
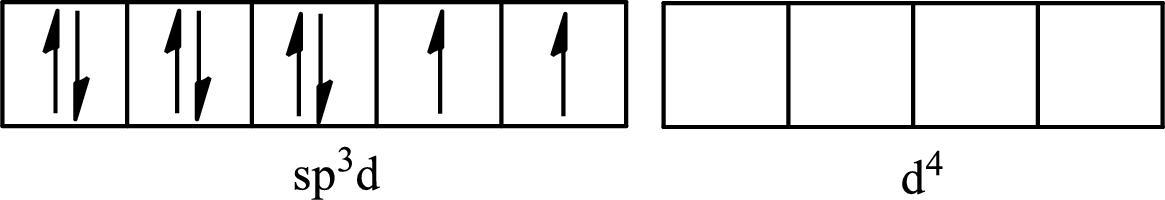
The partial orbital diagram for an isolated
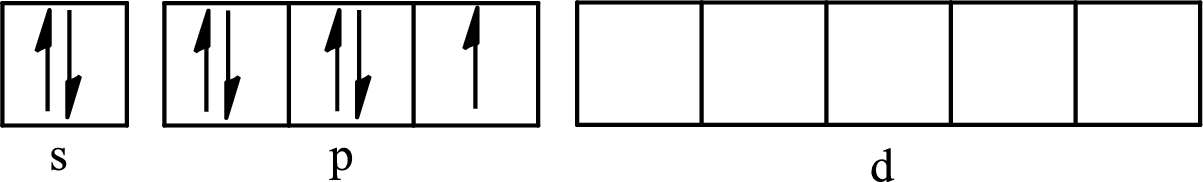
The partial orbital for hybridized
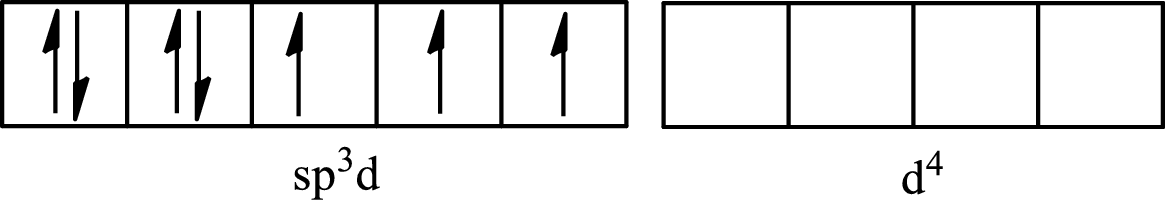
One s orbital, three p orbitals and one d orbital of central atom iodine combine to form five
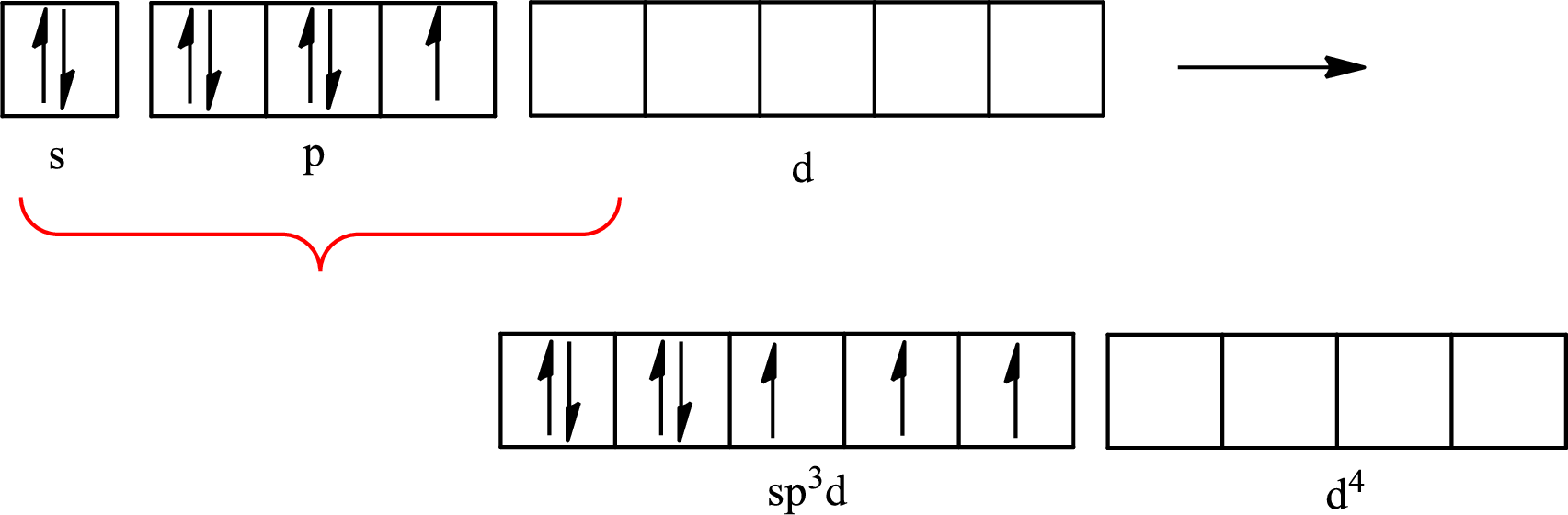
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
(c)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(c)
Answer to Problem 11.41P
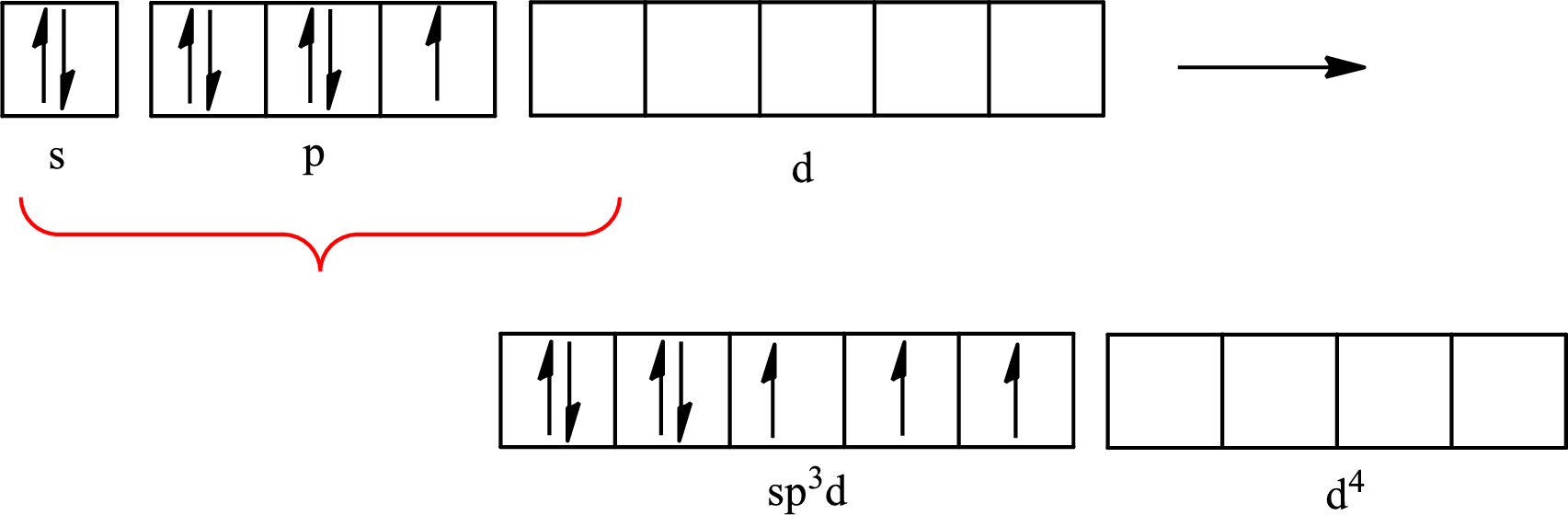
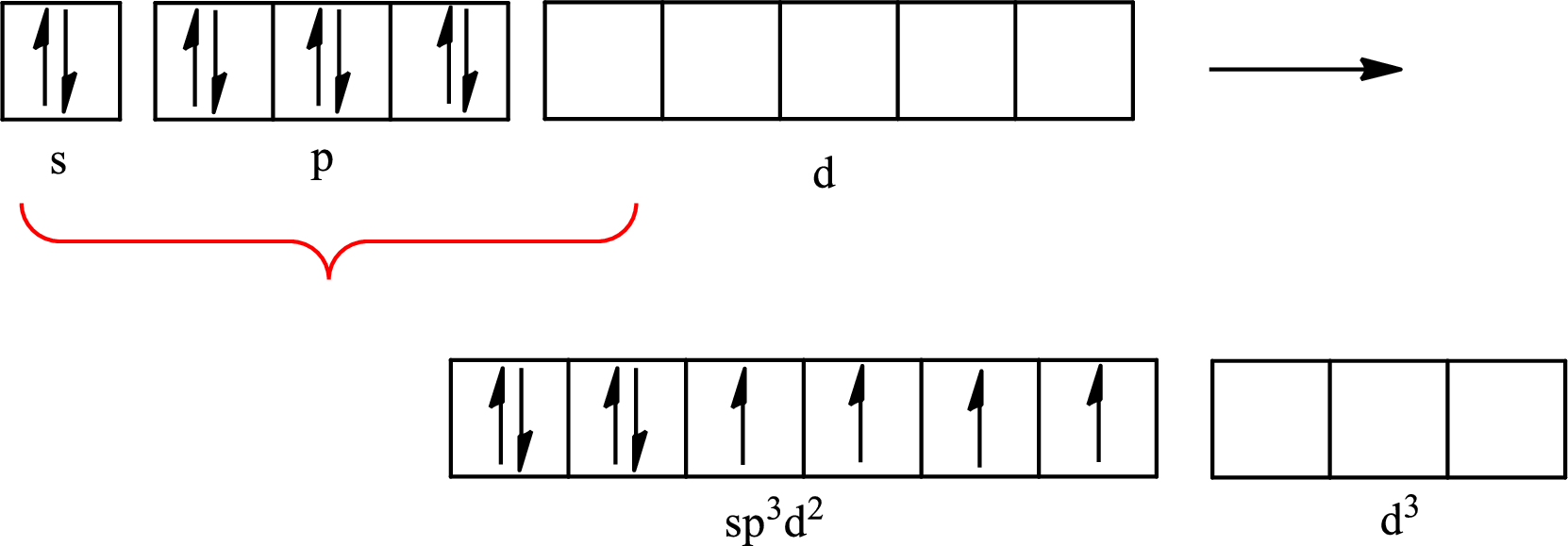
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom xenon in
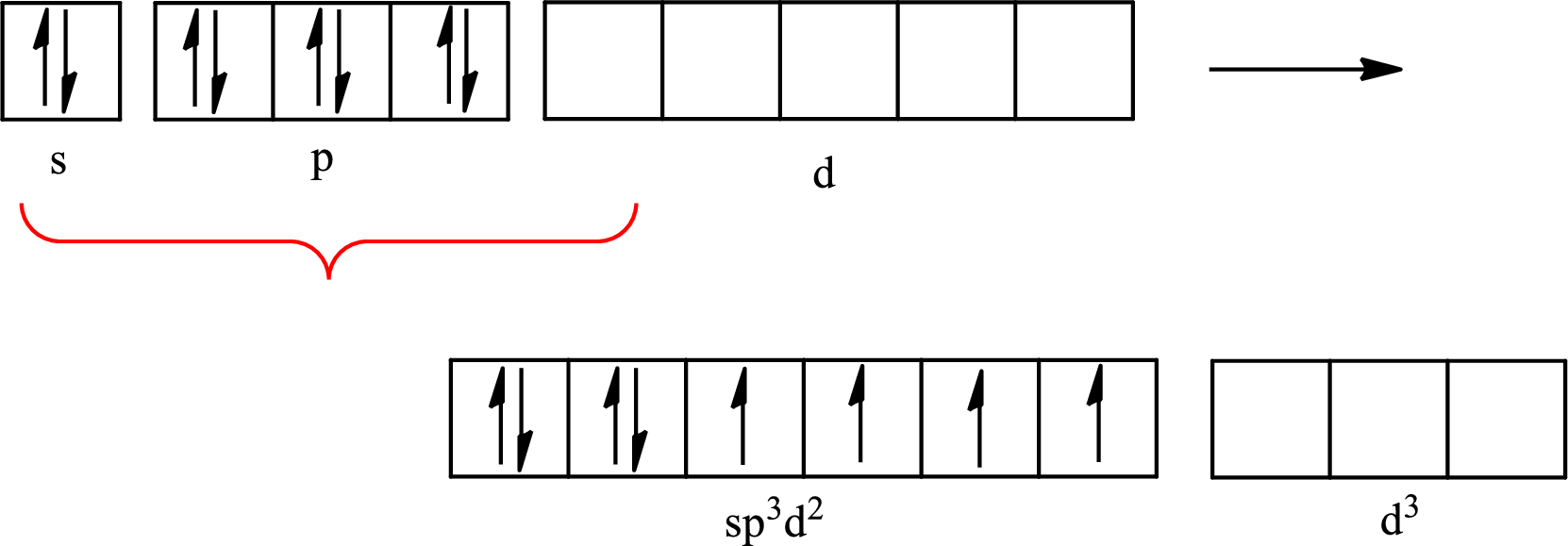
Explanation of Solution
The Lewis structure of
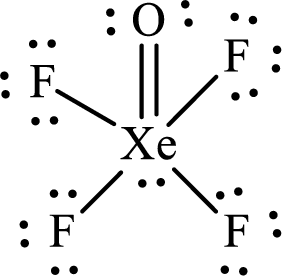
Xenon forms four single bonds with four fluorine atoms and one double bond with oxygen and one lone pair is present on it so six hybrid orbitals are required. The hybridization of xenon in
The atomic number of xenon is 54 so its electronic configuration is
The partial orbital diagram for an isolated
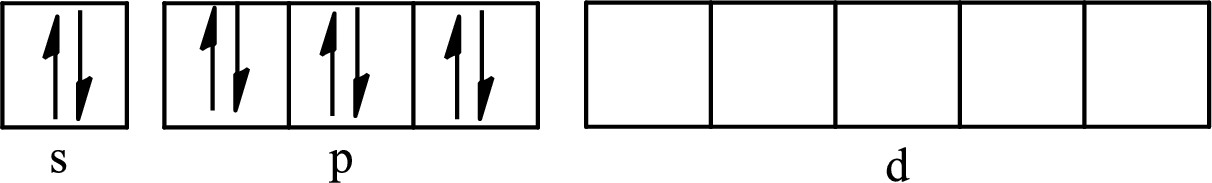
The partial orbital for hybridized
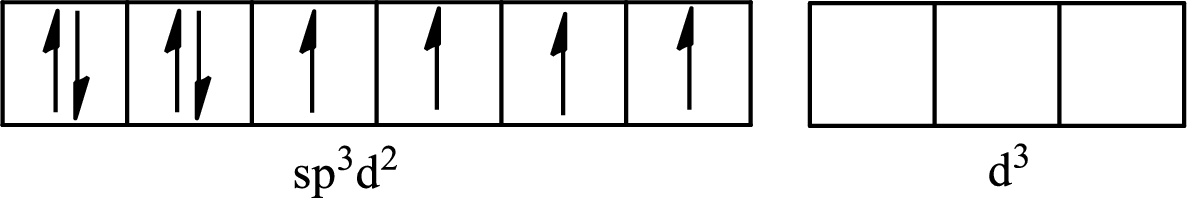
One s orbital, three p orbitals and two d orbitals of central atom xenon combine to form six
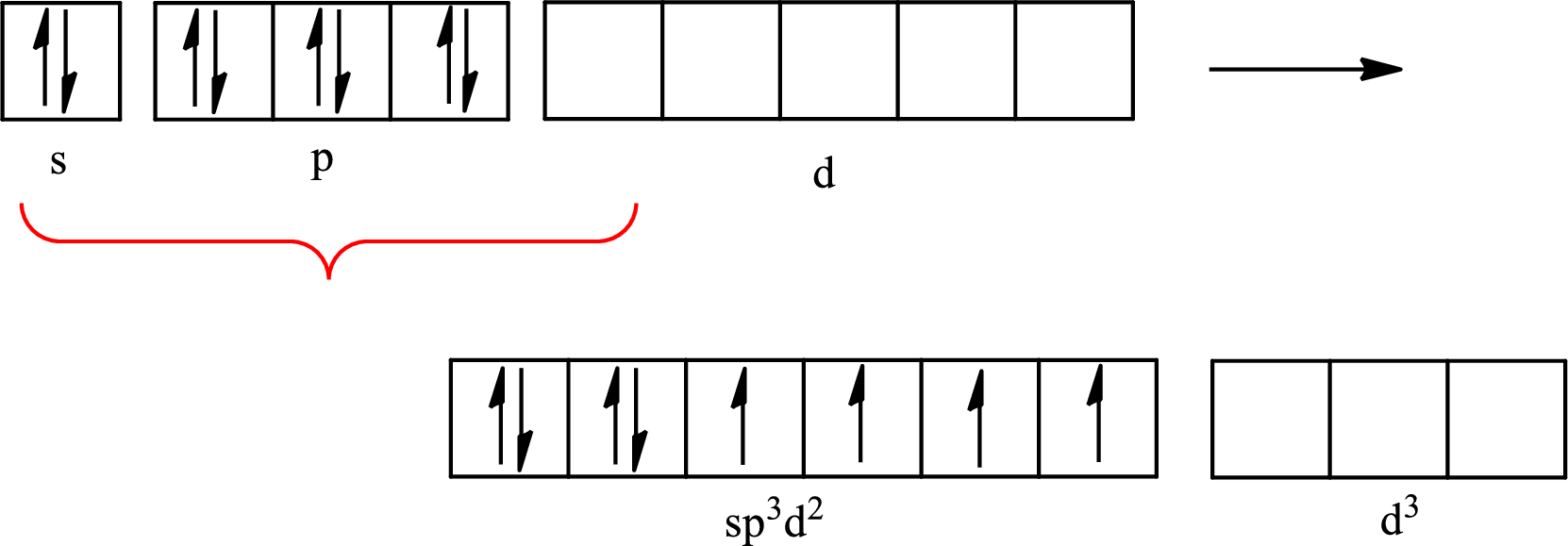
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom xenon in
(d)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(d)
Answer to Problem 11.41P
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom boron in
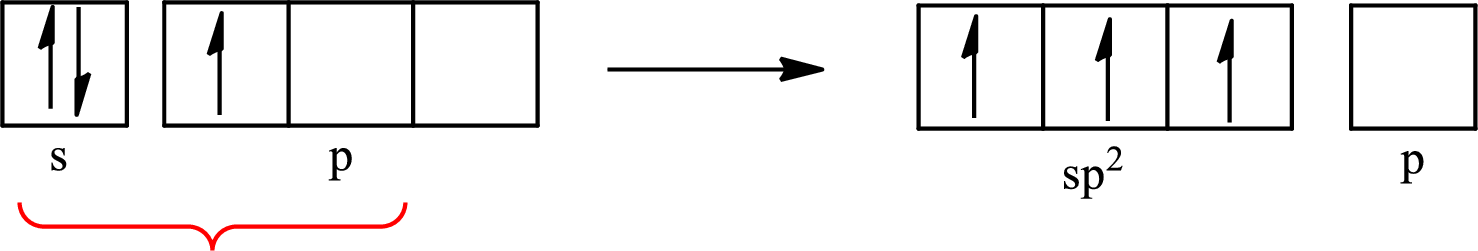
Explanation of Solution
The Lewis structure of
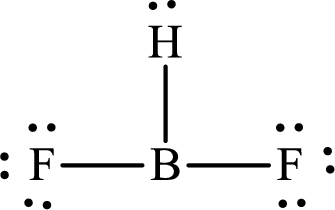
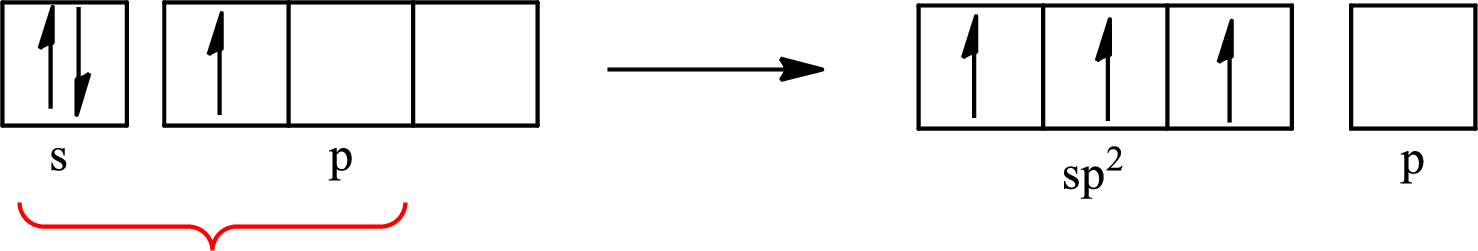
Boron forms one single bond with hydrogen and two single bonds with two fluorine atoms so three hybrid orbitals are required. The hybridization of boron in
The atomic number of boron is 5 so its electronic configuration is
The partial orbital diagram for an isolated
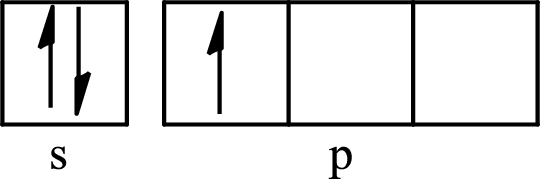
The partial orbital for hybridized
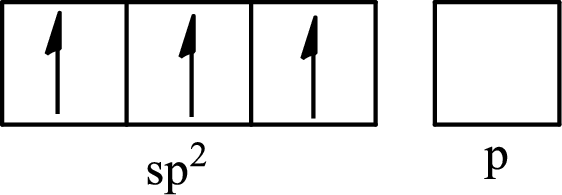
One s orbital and two p orbitals of central atom boron combine to form three
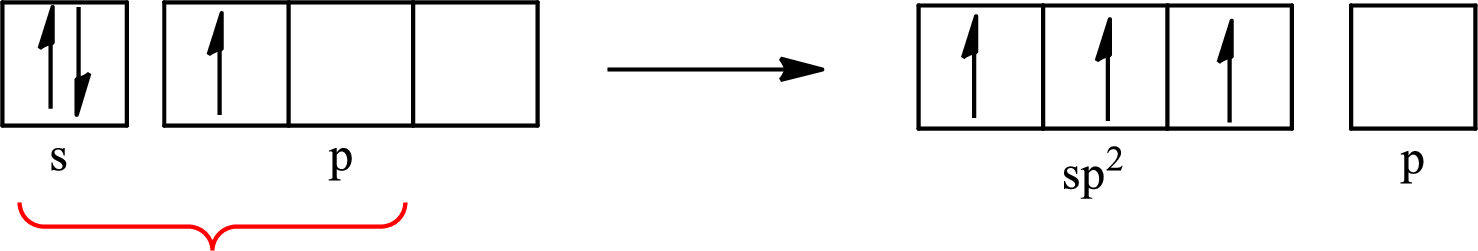
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom boron in
Want to see more full solutions like this?
Chapter 11 Solutions
Connect 2-Year Access Card for Chemistry: The Molecular Nature of Matter and Change
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





