
Concept explainers
(a)
Interpretation:
The detailed mechanism for the production of given compounds from the respective alkyne is to be drawn.
Concept introduction:
The
Answer to Problem 11.39P
The detailed mechanism for the given reaction with a major product is:
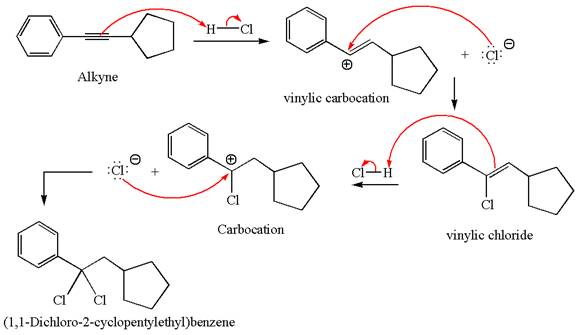
Explanation of Solution
The structure for the given compound
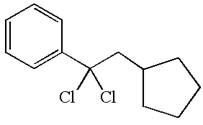
The given compound is germinal dichloride, and thus can be produced by electrophilic addition of an excess of
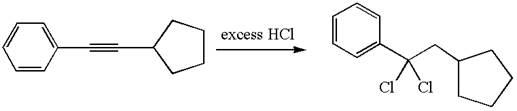
In the given reaction the alkyne is the electron rich site and the hydrogen from the
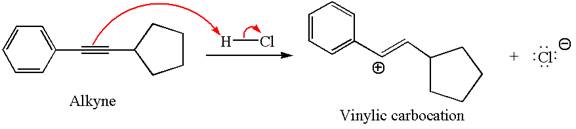
In the second step, the chloride ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic chloride product.
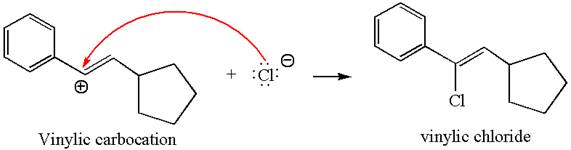
The vinylic chloride again undergoes the addition of
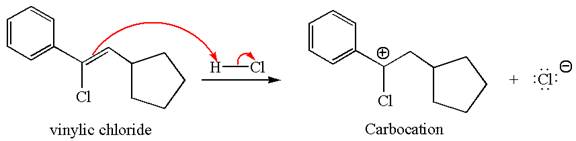
This carbocation further reacts with nucleophilic chloride ion to form a germinal dichloride product.
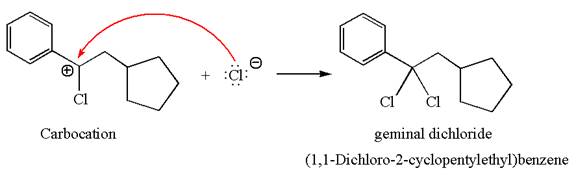
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(b)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In excess of reagent, the reaction occurs twice forming a geminal dihalide compound as a major product.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with the major product is:
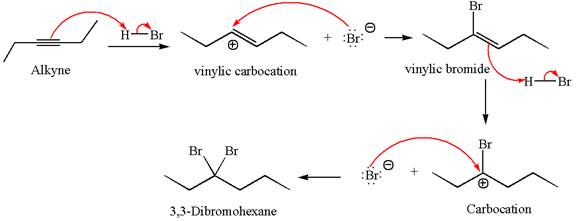
Explanation of Solution
The structure for the given compound
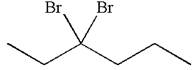
The given compound is germinal dibromide, thus can be produced by electrophilic addition of an excess of
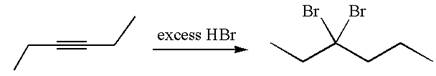
In the given reaction the alkyne is the electron rich site and the hydrogen from the
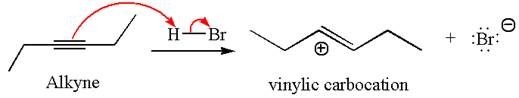
In the second step, the bromide ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic bromide product.
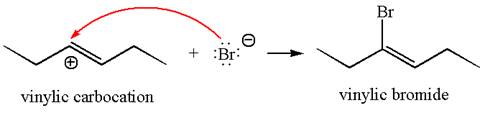
The vinylic bromide again undergoes the addition of
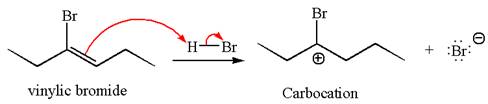
This carbocation further reacts with nucleophilic bromide ion to form germinal dibromide product.
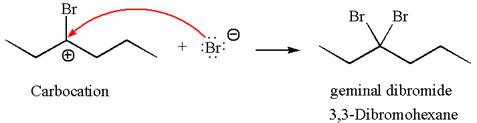
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(c)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In a single addition reaction, the reaction occurs only once forming a vibylic halide compound as a major product. The deuterium is an isotope of a hydrogen atom and reacts the same as hydrogen.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with a major product is:
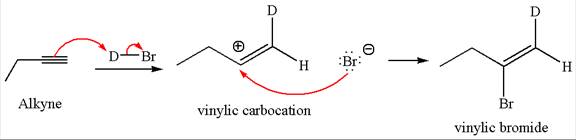
Explanation of Solution
The structure for the given compound is:
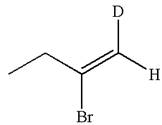
The given compound is vinylic bromide having deuterium at adjacent carbon, thus can be produced by single electrophilic addition of
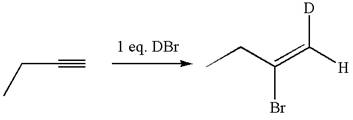
In the given reaction the alkyne is the electron rich site and the hydrogen from the
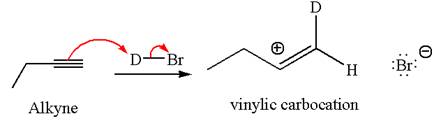
In the second step the bromide ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic bromide product.
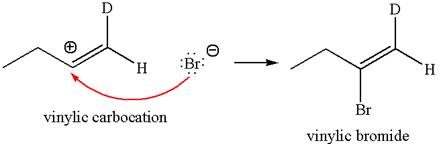
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(d)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In excess of reagent, the reaction occurs twice forming a geminal dihalide compound as a major product.
Answer to Problem 11.39P
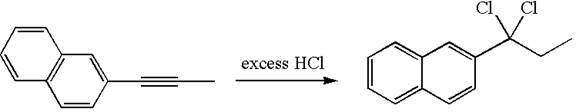
The detailed mechanism for the given reaction with the major product is:
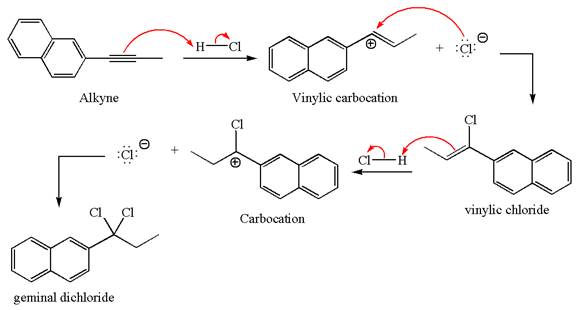
Explanation of Solution
The structure for the given compound is:
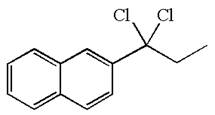
The given compound is germinal dichloride, thus can be produced by electrophilic addition of an excess of
In the given reaction the alkyne is the electron rich site and the hydrogen from the
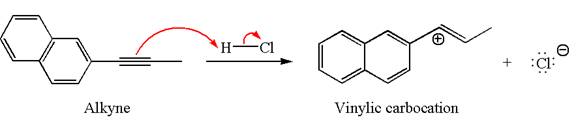
In the second step, the chloride ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic chloride products.
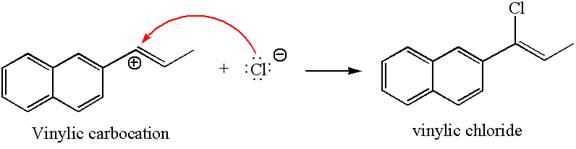
The vinylic chloride again undergoes the addition of
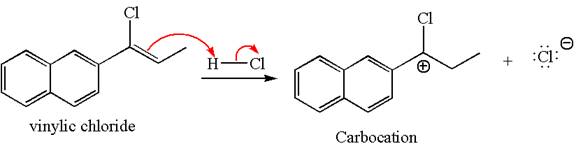
This carbocation further reacts with nucleophilic chloride ion to form a germinal dichloride product.
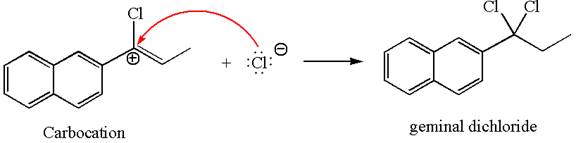
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry: Principles And Mechanisms
- CUE COLUMN NOTES (A. Determine Stereoisomers it has ⑤ Identify any meso B compounds cl Br cl -c-c-c-c-¿- 1 CI C- | 2,4-Dichloro-3-bromopentanearrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forwardWhat does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forward
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forwardIdentifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- * Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

