
Concept explainers
Intermolecular Forces
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these elements reacts with the others.
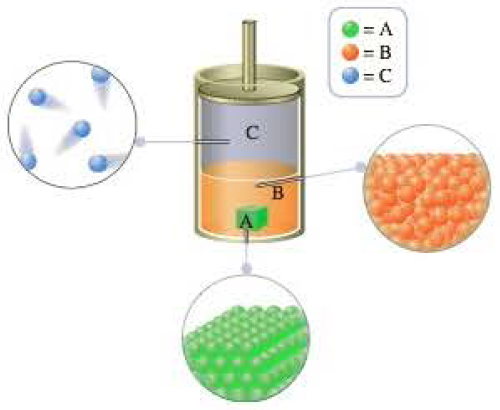
- a What is the state (solid, liquid, or gas) of each of the elements represented in the container?
- b Rank the elements in the container from greatest to least, in terms of intermolecular interactions. Explain your answer.
- c What type(s) of intermolecular attractions are present in each of these elements?
- d Explain which element has the greatest
atomic mass . - e One of the elements in the container has a normal boiling point of 2 K. Which element would that be (A, B, or C)? How do you know?
- f One of the elements has a melting point of 50 K. Which element would that be (A, B, or C)? Why?
- g The remaining element (the one you have yet to choose) has a normal boiling point of 25 K. Identify the element. Could this element have a freezing point of 7 K? Explain.
- h If you started heating the sample to 20 K, explain what you would observe with regard to the container and its contents during the heating.
- i Describe the container and its contents at 20 K. Describe (include a drawing) how the container and its contents look at 20 K.
- j Now you increase the temperature of the container to 30 K. Describe (include a drawing) how the container and its contents look at 30 K. Be sure to note any changes in going from 20 K to 30 K.
- k Finally, you heat the container to 60 K. Describe (include a drawing) how the container and its contents look at this temperature. Be sure to note any changes in going from 30K to 60k
(a)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
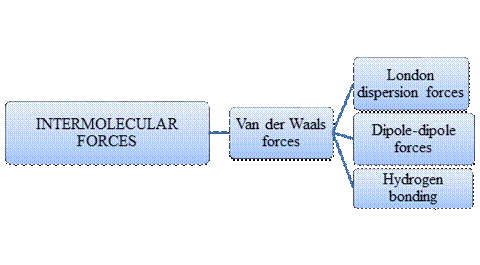
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: the state of each of the elements in the vessel
In a vessel, A is in solid state, B is in liquid state and C is in gaseous state.
Because of solids have definite shape and volume, liquids are definite volume and indefinite shape and gases have both indefinite volume and shape.
(b)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To rank: the elements from highest to lowest intermolecular attraction
Solids have highest intermolecular attractions than liquids which has greater intermolecular attraction than gases.
Thus, the increasing order of intermolecular attractions is,
(c)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: intermolecular attractions in each of these elements
Each of the substances are found to be monoatomic non-metal. So London forces only present.
(d)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: the element has highest atomic mass
The highest atomic mass will be for highly stronger intermolecular forces. Hence, which is found to be A (solid).
(e)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the element has normal boiling point of 2K
The element with normal boiling point of 2K is found to be gas (C). Because of gas is substance whose boiling point is lower than ambient temperature.
(f)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the element has melting point of 50K
The substance with melting point of 50K is found to be A (solid). Because of solids must have melting point be higher than ambient temperature.
(g)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the remaining element has normal boiling point of 25K
The substance with normal boiling point of 25K is found to be B. Because it cannot freeze at 7K or it should be in solid state. The demand for a substance to be a liquid, the freezing point should be lower than ambient temperature.
(h)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: what happen when sample is heated to 20K regards to vessel
As we start to heat the vessel to 20K, the gas should expand and the piston would move awake.
(i)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: the container and its content at
At
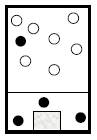
Figure 1
(j)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: how container and its content at 30K and ant changes from 20K to 30K
The temperature is now higher than the boiling point of B which is 25K; therefore both B and C are now gaseous phase. A persists in solid state.
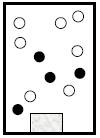
Figure 2
(k)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: how container and its content at 60K and ant changes from 30K to 60K
When the temperature of the container attains 60K, the component A has now melted and changed into liquid phase. The gases B and C are continue to enlarge according to Charles’s law.
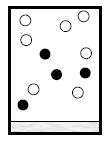
Figure 3
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: General Chemistry, Loose-leaf Version, 11th + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





