![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months)](https://www.bartleby.com/isbn_cover_images/9781305864900/9781305864900_largeCoverImage.jpg)
Concept explainers
Intermolecular Forces
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these elements reacts with the others.
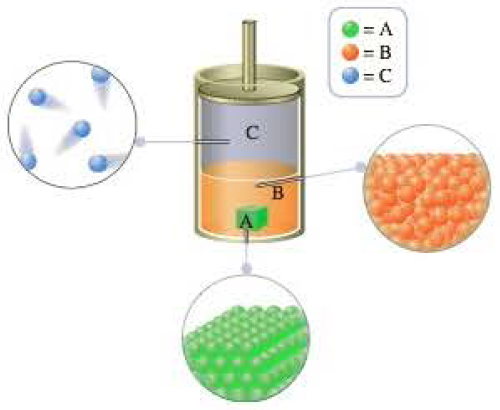
- a What is the state (solid, liquid, or gas) of each of the elements represented in the container?
- b Rank the elements in the container from greatest to least, in terms of intermolecular interactions. Explain your answer.
- c What type(s) of intermolecular attractions are present in each of these elements?
- d Explain which element has the greatest
atomic mass . - e One of the elements in the container has a normal boiling point of 2 K. Which element would that be (A, B, or C)? How do you know?
- f One of the elements has a melting point of 50 K. Which element would that be (A, B, or C)? Why?
- g The remaining element (the one you have yet to choose) has a normal boiling point of 25 K. Identify the element. Could this element have a freezing point of 7 K? Explain.
- h If you started heating the sample to 20 K, explain what you would observe with regard to the container and its contents during the heating.
- i Describe the container and its contents at 20 K. Describe (include a drawing) how the container and its contents look at 20 K.
- j Now you increase the temperature of the container to 30 K. Describe (include a drawing) how the container and its contents look at 30 K. Be sure to note any changes in going from 20 K to 30 K.
- k Finally, you heat the container to 60 K. Describe (include a drawing) how the container and its contents look at this temperature. Be sure to note any changes in going from 30K to 60k
(a)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 1](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image001.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: the state of each of the elements in the vessel
In a vessel, A is in solid state, B is in liquid state and C is in gaseous state.
Because of solids have definite shape and volume, liquids are definite volume and indefinite shape and gases have both indefinite volume and shape.
(b)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 2](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image002.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To rank: the elements from highest to lowest intermolecular attraction
Solids have highest intermolecular attractions than liquids which has greater intermolecular attraction than gases.
Thus, the increasing order of intermolecular attractions is,
(c)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 3](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image003.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: intermolecular attractions in each of these elements
Each of the substances are found to be monoatomic non-metal. So London forces only present.
(d)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 4](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image004.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: the element has highest atomic mass
The highest atomic mass will be for highly stronger intermolecular forces. Hence, which is found to be A (solid).
(e)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 5](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image004.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the element has normal boiling point of 2K
The element with normal boiling point of 2K is found to be gas (C). Because of gas is substance whose boiling point is lower than ambient temperature.
(f)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 6](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image002.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the element has melting point of 50K
The substance with melting point of 50K is found to be A (solid). Because of solids must have melting point be higher than ambient temperature.
(g)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 7](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image003.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the remaining element has normal boiling point of 25K
The substance with normal boiling point of 25K is found to be B. Because it cannot freeze at 7K or it should be in solid state. The demand for a substance to be a liquid, the freezing point should be lower than ambient temperature.
(h)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 8](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image004.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: what happen when sample is heated to 20K regards to vessel
As we start to heat the vessel to 20K, the gas should expand and the piston would move awake.
(i)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 9](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image004.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: the container and its content at
At
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 10](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image005.jpg)
Figure 1
(j)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 11](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image001.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: how container and its content at 30K and ant changes from 20K to 30K
The temperature is now higher than the boiling point of B which is 25K; therefore both B and C are now gaseous phase. A persists in solid state.
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 12](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image006.jpg)
Figure 2
(k)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 13](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image002.gif)
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: how container and its content at 60K and ant changes from 30K to 60K
When the temperature of the container attains 60K, the component A has now melted and changed into liquid phase. The gases B and C are continue to enlarge according to Charles’s law.
![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months), Chapter 11, Problem 11.25QP , additional homework tip 14](https://content.bartleby.com/tbms-images/9781305580343/Chapter-11/images/80343-11-11.25qp_image007.jpg)
Figure 3
Want to see more full solutions like this?
Chapter 11 Solutions
OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months)
- What is the lone pair or charge that surrounds the nitrogen here to give it that negative charge?arrow_forwardLast Name, Firs Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $ 4th total 21 total 4H total ZH 2H Statistical H < 3°C-H werkst - product bund abstraction here leads to the mo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Proclict 6 Number of Unique Mono-Chlorinated Products f Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary 'H H-Cl Waterfoxarrow_forward2. (a) Many main group oxides form acidic solutions when added to water. For example solid tetraphosphorous decaoxide reacts with water to produce phosphoric acid. Write a balanced chemical equation for this reaction. (b) Calcium phosphate reacts with silicon dioxide and carbon graphite at elevated temperatures to produce white phosphorous (P4) as a gas along with calcium silicate (Silcate ion is SiO3²-) and carbon monoxide. Write a balanced chemical equation for this reaction.arrow_forward
- this is an organic chemistry question please answer accordindly!! please post the solution in your hand writing not an AI generated answer please draw the figures and structures if needed to support your explanation hand drawn only!!!! answer the question in a very simple and straight forward manner thanks!!!!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forward2B: The retrosynthetic cut below provides two options for a Suzuki coupling, provide the identities of A, B, C and D then identify which pairing is better and justify your choice. O₂N. Retro-Suzuki NO2 MeO OMe A + B OR C + Darrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution in your hand writing not an AI generated answer please draw the figures and structures if needed to support your explanation hand drawn only!!!! answer the question in a very simple and straight forward manner thanks!!!!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





