
Chemistry: Structure and Properties, Books a la Carte PACKAGE W/MasteringChemistry, 2nd Edition
2nd Edition
ISBN: 9780134777559
Author: Tro, Nivaldo J.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 110E
Interpretation Introduction
Interpretation:
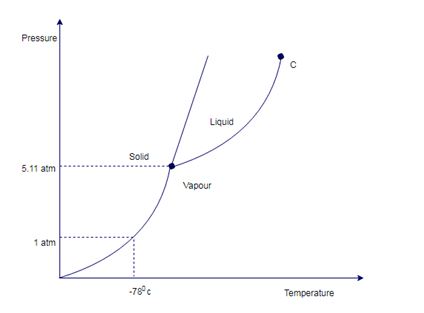
Sketch the phase diagram of carbon dioxide. It may or may not be possible to turn it into liquid by cooling it down at 1.0 atm and 25° C.
Concept introduction:
A pure substance generally has three phases at different condition. Representation of all the phases at different condition form phase diagram.
Phase diagram for carbon dioxide
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10.
Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the
following compound. In order to recieve full credit, you MUST SHOW YOUR WORK!
H₂N
CI
OH
CI
カー
11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign
R/S configurations for all stereoisomers and indicate the relationship between each as
enantiomer, diastereomer, or meso.
NH2
H
HNH,
-18
b)
8.
Indicate whether the following carbocation rearrangements are likely to occur
Please explain your rational using 10 words or less
not likely to occur
• The double bond is still in the
Same position
+
Likely
to oc
occur
WHY?
-3
H3C
Brave
Chair Conformers. Draw the chair conformer of the following substituted
cyclohexane. Peform a RING FLIP and indicate the most stable
conformation and briefly explain why using 20 words or less.
CI
2
-cobs ??
MUST INDICATE H -2
-2
Br
EQ
Cl
OR
AT
Br
H&
most stable
WHY?
- 4
CH
12
Conformational Analysis. Draw all 6 conformers (one above each letter) of the
compound below looking down the indicated bond. Write the letter of the
conformer with the HIGHEST and LOWEST in energies on the lines provided.
NOTE: Conformer A MUST be the specific conformer of the structure as drawn below
-4 NOT
HOH
OH
3
Conformer A:
Br
OH
A
Samo
Br H
04
Br
H
H3
CH₂
H
anti
stagere
Br CH
clipsed
H
Brott
H
IV
H
MISSING 2
-2
B
C
D
E
F
X
6
Conformer with HIGHEST ENERGY:
13. (1
structure
LOWEST ENERGY:
Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the
corresponding to this name. HINT: Do not forget to indicate stereochemistry
when applicable.
a)
८८
2
"Br
{t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa
Parent name (noname)
4 Bromo
Sub = 2-methylethyl-4 Bromo nonane
b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane
# -2
-2
Chapter 11 Solutions
Chemistry: Structure and Properties, Books a la Carte PACKAGE W/MasteringChemistry, 2nd Edition
Ch. 11 - Why do ethanol and dimethyl ether have such...Ch. 11 - Why are intermolecular forces important?Ch. 11 - Prob. 3ECh. 11 - Prob. 4ECh. 11 - Prob. 5ECh. 11 - Which factors cause transitions between the solid...Ch. 11 - Describe the relationship between the state of a...Ch. 11 - Prob. 8ECh. 11 - Prob. 9ECh. 11 - Prob. 10E
Ch. 11 - Prob. 11ECh. 11 - Prob. 12ECh. 11 - Prob. 13ECh. 11 - What is the ion-dipole force? Why is it important?Ch. 11 - Prob. 15ECh. 11 - Prob. 16ECh. 11 - What is capillary action? How does it depend on...Ch. 11 - Explain what happens during the processes of...Ch. 11 - Why is vaporization endothermic? Why is...Ch. 11 - Prob. 20ECh. 11 - What is the heat of vaporization for a liquid, and...Ch. 11 - Explain the process of dynamic equilibrium. How is...Ch. 11 - What happens to a system in dynamic equilibrium...Ch. 11 - Prob. 24ECh. 11 - Prob. 25ECh. 11 - Prob. 26ECh. 11 - Prob. 27ECh. 11 - Prob. 28ECh. 11 - Prob. 29ECh. 11 - Prob. 30ECh. 11 - Prob. 31ECh. 11 - Examine the heating curve for water in section...Ch. 11 - What is a phase diagram? What is the significance...Ch. 11 - Draw a generic phase diagram and label its...Ch. 11 - Prob. 35ECh. 11 - Determine the kinds of intermolecular forces that...Ch. 11 - Determine the kinds of intermolecular forces that...Ch. 11 - Prob. 38ECh. 11 - Arrange these compounds in order of increasing...Ch. 11 - Prob. 40ECh. 11 - Pick the compound with the highest boiling point...Ch. 11 - Pick the compound with the highest boiling point...Ch. 11 - Prob. 43ECh. 11 - Prob. 44ECh. 11 - Prob. 45ECh. 11 - Prob. 46ECh. 11 - Prob. 47ECh. 11 - Water (a) “wets” some surfaces and beads up on...Ch. 11 - The structures of two isomers of heptanes are...Ch. 11 - Prob. 50ECh. 11 - Water in a glass tube that contains grease or oil...Ch. 11 - When a thin glass tube is put into water, the...Ch. 11 - Which evaporates more quickly: 55 mL of water in a...Ch. 11 - Prob. 54ECh. 11 - Spilling room temperature water over your skin on...Ch. 11 - Prob. 56ECh. 11 - The human body obtains 915 kJ of energy from a...Ch. 11 - Prob. 58ECh. 11 - Suppose that 0.95 g of water condenses on a 75.0 g...Ch. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Prob. 62ECh. 11 - Prob. 63ECh. 11 - Prob. 64ECh. 11 - How much energy is released when 65.8 g of water...Ch. 11 - Prob. 66ECh. 11 - An 8.5 g ice cube is placed into 255 g of water....Ch. 11 - Prob. 68ECh. 11 - Prob. 69ECh. 11 - Prob. 70ECh. 11 - Prob. 71ECh. 11 - Prob. 72ECh. 11 - Prob. 73ECh. 11 - Prob. 74ECh. 11 - Prob. 75ECh. 11 - The high-pressure phase diagram of ice is shown...Ch. 11 - Prob. 77ECh. 11 - Prob. 78ECh. 11 - Prob. 79ECh. 11 - How is the density of solid water compared to that...Ch. 11 - Prob. 81ECh. 11 - Prob. 82ECh. 11 - Prob. 83ECh. 11 - Prob. 84ECh. 11 - Four ice cubes at exactly 00C with a total mass of...Ch. 11 - Prob. 86ECh. 11 - Draw a heating curve (such as the one in Figure...Ch. 11 - Draw a heating curve (such as the one in Figure...Ch. 11 - Prob. 89ECh. 11 - A sealed flask contains 0.55 g of water at 280C....Ch. 11 - Prob. 91ECh. 11 - Prob. 92ECh. 11 - Prob. 93ECh. 11 - Given that the heat of fusion of water is —6.02...Ch. 11 - The heat of combustion of CH4 is 890.4 kJ/mol, and...Ch. 11 - Prob. 96ECh. 11 - Prob. 97ECh. 11 - Prob. 98ECh. 11 - Prob. 99ECh. 11 - Prob. 100ECh. 11 - Prob. 101ECh. 11 - Prob. 102ECh. 11 - Prob. 103ECh. 11 - Prob. 104ECh. 11 - Prob. 105ECh. 11 - A substance has a triple point at a temperature of...Ch. 11 - The boiling of three compounds are tabulated here....Ch. 11 - Prob. 108ECh. 11 - Based on the heating curve for water, does it take...Ch. 11 - Prob. 110ECh. 11 - Prob. 111ECh. 11 - Prob. 1SAQCh. 11 - Liquid nitrogen boils at 77 K. This image depicts...Ch. 11 - Taking intermolecular forces into account, which...Ch. 11 - What substance experiences dipole-dipole forces?...Ch. 11 - Prob. 5SAQCh. 11 - Prob. 6SAQCh. 11 - Determine the amount of heat (in kJ) required to...Ch. 11 - Prob. 8SAQCh. 11 - Prob. 9SAQCh. 11 - Prob. 10SAQCh. 11 - Prob. 11SAQCh. 11 - Determine which state this substance is in at 1...Ch. 11 - Prob. 13SAQ
Knowledge Booster
Similar questions
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning