![WebAssign for Zumdahl's Chemical Principles, 8th Edition [Instant Access], Single-Term](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
WebAssign for Zumdahl's Chemical Principles, 8th Edition [Instant Access], Single-Term
8th Edition
ISBN: 9780357119112
Author: Zumdahl; Steven S.
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 10DQ
What is the difference between 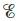 and
and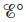 When is
When is 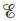 equal to zero? When is
equal to zero? When is 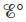 equal to zero? (Consider“regular” galvanic cells as well as concentration cells.)
equal to zero? (Consider“regular” galvanic cells as well as concentration cells.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using Benzene as starting materia Show
how each of the Following molecules could
Ve synthesked
9.
CHI
d.
10450
b
0
-50311
८
City
-5034
1-0-650
e
NO2
BA
HBr
of the fol
1)=MgCI
2) H₂O
major
NaOEt
Ts Cl
Py (pyridine)
1) 03
2) Me2S
1
4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a)
NHBoc
⚫OBn
HO.
H3C
CO2CH3
-OBn
H3C
H3C.
H3C.
NHBOC
CI
CO2CH3
Chapter 11 Solutions
WebAssign for Zumdahl's Chemical Principles, 8th Edition [Instant Access], Single-Term
Ch. 11 - Prob. 1DQCh. 11 - Prob. 2DQCh. 11 - You want to “plate out” nickel metal from a nickel...Ch. 11 - A copper penny can be dissolved in nitric acid but...Ch. 11 - Sketch a cell that forms iron metal from iron(II)...Ch. 11 - Which of the following is the best reducing agent:...Ch. 11 - You are told that metal A is a better reducing...Ch. 11 - Explain the following relationships: G and w, cell...Ch. 11 - Explain why cell potentials are not multiplied by...Ch. 11 - What is the difference between andWhen is equal to...
Ch. 11 - Prob. 11DQCh. 11 - Look up the reduction potential for Fe3+toFe2+ ....Ch. 11 - Prob. 13DQCh. 11 - Is the following statement true or false?...Ch. 11 - What is electrochemistry? What are redox...Ch. 11 - When magnesium metal is added to a beaker of...Ch. 11 - Prob. 17ECh. 11 - How can you construct a galvanic cell from two...Ch. 11 - Prob. 19ECh. 11 - Prob. 20ECh. 11 - Prob. 21ECh. 11 - Consider the following galvanic cells: For each...Ch. 11 - Prob. 23ECh. 11 - Prob. 24ECh. 11 - Answer the following questions using data from...Ch. 11 - Prob. 26ECh. 11 - Using data from Table 11.1, place the following in...Ch. 11 - Prob. 28ECh. 11 - Use the table of standard reduction potentials...Ch. 11 - Use the table of standard reduction potentials...Ch. 11 - Prob. 31ECh. 11 - A patent attorney has asked for your advice...Ch. 11 - The free energy change for a reaction G is an...Ch. 11 - The equation also can be applied to...Ch. 11 - Prob. 35ECh. 11 - Glucose is the major fuel for most living cells....Ch. 11 - Direct methanol fuel cells (DMFCs) have shown...Ch. 11 - The overall reaction and standard cell potential...Ch. 11 - Calculate the maximum amount of work that can...Ch. 11 - Prob. 40ECh. 11 - Prob. 41ECh. 11 - Chlorine dioxide (ClO2) , which is produced by...Ch. 11 - The amount of manganese in steel is determined...Ch. 11 - The overall reaction and equilibrium constant...Ch. 11 - Prob. 45ECh. 11 - Calculate for the reaction...Ch. 11 - A disproportionation reaction involves a substance...Ch. 11 - Calculate for the following half-reaction:...Ch. 11 - For the following half-reaction AlF63+3eAl+6F...Ch. 11 - Prob. 50ECh. 11 - The solubility product for CuI(s) is 1.11012....Ch. 11 - Explain the following statement: determines...Ch. 11 - Calculate the pH of the cathode compartment for...Ch. 11 - Consider the galvanic cell based on the...Ch. 11 - Prob. 55ECh. 11 - Consider the following galvanic cell at 25°C:...Ch. 11 - The black silver sulfide discoloration of...Ch. 11 - Consider the cell described below:...Ch. 11 - Consider the cell described below:...Ch. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Prob. 62ECh. 11 - What are concentration cells? What is in a...Ch. 11 - A silver concentration cell is set up at 25°C as...Ch. 11 - Consider the concentration cell shown below....Ch. 11 - Prob. 66ECh. 11 - Prob. 67ECh. 11 - An electrochemical cell consists of a nickel metal...Ch. 11 - You have a concentration cell in which the cathode...Ch. 11 - Consider a galvanic cell at standard conditions...Ch. 11 - An electrochemical cell consists of a zinc metal...Ch. 11 - How long will it take to plate out each of the...Ch. 11 - What mass of each of the following substances can...Ch. 11 - It took 2.30 min with a current of 2.00 A to plate...Ch. 11 - The electrolysis of BiO+ produces pure bismuth....Ch. 11 - A single HallHeroult cell (as shown in Fig. 11.22)...Ch. 11 - A factory wants to produce 1.00103 kg barium...Ch. 11 - Why is the electrolysis of molten salts much...Ch. 11 - What reaction will take place at the cathode and...Ch. 11 - What reaction will take place at the cathode and...Ch. 11 - Prob. 81ECh. 11 - a. In the electrolysis of an aqueous solution of...Ch. 11 - A solution at 25°C contains 1.0 M...Ch. 11 - An aqueous solution of an unknown salt of...Ch. 11 - Consider the following half-reactions: A...Ch. 11 - An unknown metal M is electrolyzed. It took 74.1 s...Ch. 11 - Electrolysis of an alkaline earth metal chloride...Ch. 11 - Prob. 88ECh. 11 - What volume of F2 gas, at 25°C and 1.00 atm, is...Ch. 11 - Prob. 90ECh. 11 - In the electrolysis of a sodium chloride solution,...Ch. 11 - What volumes of H2(g)andO2(g) at STP are...Ch. 11 - Copper can be plated onto a spoon by placing the...Ch. 11 - Prob. 94AECh. 11 - Prob. 95AECh. 11 - Prob. 96AECh. 11 - Prob. 97AECh. 11 - Prob. 98AECh. 11 - Prob. 99AECh. 11 - Prob. 100AECh. 11 - Prob. 101AECh. 11 - Prob. 102AECh. 11 - Prob. 103AECh. 11 - Prob. 104AECh. 11 - In 1973 the wreckage of the Civil War ironclad...Ch. 11 - A standard galvanic cell is constructed so that...Ch. 11 - Prob. 107AECh. 11 - Prob. 108AECh. 11 - Prob. 109AECh. 11 - Prob. 110AECh. 11 - Prob. 111AECh. 11 - Prob. 112AECh. 11 - Prob. 113AECh. 11 - Consider a galvanic cell based on the following...Ch. 11 - Prob. 115AECh. 11 - Prob. 116AECh. 11 - Prob. 117AECh. 11 - Prob. 118AECh. 11 - Prob. 119CPCh. 11 - Prob. 120CPCh. 11 - A zinccopper battery is constructed as follows:...Ch. 11 - Prob. 122CPCh. 11 - Prob. 123CPCh. 11 - Prob. 124CPCh. 11 - Prob. 125CPCh. 11 - Prob. 126CPCh. 11 - Prob. 127CPCh. 11 - Prob. 128CPCh. 11 - Prob. 129CPCh. 11 - Prob. 130CPCh. 11 - Prob. 131CPCh. 11 - Prob. 132MPCh. 11 - Prob. 133MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward1) NaNH2 (excess) 1) NaNH2 CI CI 2) H₂O 2) Mel 1) 03 2) (CH3)2S Na NH3 (liquid) 1arrow_forward
- CI 1) n-BuLi 2) 1) 03 HH T&Cl 2) H₂O 2arrow_forwardHelp with a!arrow_forwardFor the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forward
- identify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forward
- For the following compound: HO H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: + H-O: H-OH₂ H Mechanism B: H-Ö: HO-H H-OO -H H HH H H HH H-O: H-OO H-OO -H H e -H : OH Θ Mechanism C: Θ A : OH H-O: H H H-O-H 0. Mechanism D: e.. : OH :0 H H-O-H H-O: H-OO :O H -H H H сём H 0 :0 + H Θ H H H-arrow_forwardFor the following compound: H OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Θ :OH O O-H HO 0: Mechanism B: :O-H e.. Θ :OH Mechanism C: H HO-H :0: Θ 0: H H e.. : OH 0: "Θ HH O. :OH :OH O-H O-H Mechanism D: :OH H-OH₂ :OH HO-H 0: © O-H H HH 0: HHarrow_forwardHelp w c!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY