
a)
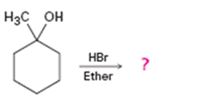
Interpretation:
The product of the reaction shown is to be predicted.
Concept introduction:
Alcohols gets converted into the corresponding bromides on treatment with HBr in ether solution.
To predict:
The product of the reaction shown.
Answer to Problem 26AP
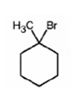
The product of the reaction shown is
Explanation of Solution
When 1-methylcyclohexanol is treated with HBr, the OH group in it is replaced by the bromine to yield 1-chloro-1-methylcyclohexane and water as products.
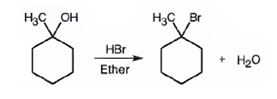
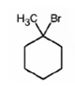
The product of the reaction shown is
b)
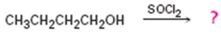
Interpretation:
The product of the reaction shown is to be predicted.
Concept introduction:
Alcohols when treated with SOCl2 yield the corresponding alkyl chlorides along with SO2 and HCl.
To predict:
The product of the reaction in which 1-butanol reacts with SOCl2 .
Answer to Problem 26AP
The product of the reaction in which 1-butanol reacts with SOCl2 is 1-chlorobutane.
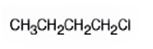
Explanation of Solution
When 1-butanol reacts with SOCl2, the OH group in is replaced by chlorine to yield 1-chlorobutane. SO2 and HCl are obtained as other products.
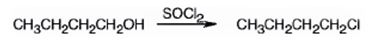
The product of the reaction in which 1-butanol reacts with SOCl2 is 1-chlorobutane.
c)
Interpretation:
The product of the reaction shown is to be predicted.
Concept introduction:
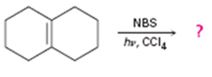
Cycloalkenes on treatment with NBS in CCl4 in the presence of light undergo bromination at the allyl position.
To predict:
The product of the reaction shown.
Answer to Problem 26AP
The products of the reaction shown are
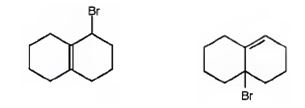
Explanation of Solution
NBS is normally used for allylic bromination. The reaction occurs through a radical mechanism. The radical produced by the homolytic cleavage of the C-H bond in the allyl position gets delocalized with the π electrons of the double bond to yield another radical with an odd electron at the junction of the rings. The attack of bromine radical hence leads to two different products as shown.
The products of the reaction shown are

d)
Interpretation:
The product of the reaction shown is to be predicted.
Concept introduction:
Alcohols give the corresponding bromides as products on treatment with PBr3 in ether solution.
To predict:
The product of the reaction in which cyclohexanol reacts with PBr3.
Answer to Problem 26AP
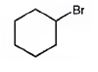
The product of the reaction in which cyclohexanol reacts with PBr3is
Explanation of Solution
When cyclohexanol is treated with PBr3, the OH group in it is replaced by the bromine to yield bromocyclohexane.
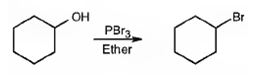
The product of the reaction in which cyclohexanol reacts with PBr3 is

e)
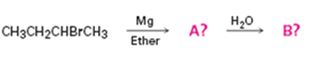
Interpretation:
The products of the reactions shown are to be predicted.
Concept introduction:
Alkyl bromides react with Mg in ether to give Grignard reagents. Grignard reagents when treated with water yield the corresponding
To predict:
The products of the reaction shown.
Answer to Problem 26AP
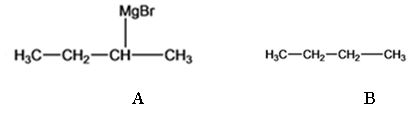
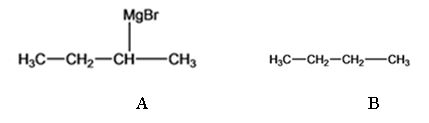
The products of the reaction shown are sec-butyl magnesium bromide (A) and n-butane (B).
Explanation of Solution
sec-butyl bromide when reacted with Mg in ether yields sec-butyl magnesium bromide, a Grignard reagent. The sec-butyl magnesium bromide reacts with water to yield n-butane.
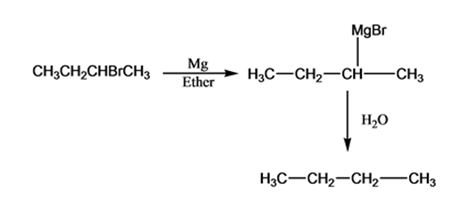
The products of the reaction shown are sec-butyl magnesium bromide (A) and n-butane (B).
f)
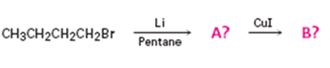
Interpretation:
The products of the reaction shown are to be predicted.
Concept introduction:
To predict:
The product of the reaction shown.
Answer to Problem 26AP
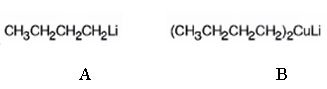
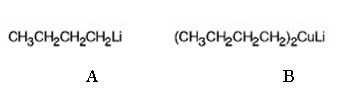
The products of the reaction shown are N-butyllithium (A) and Lithium n-dibutyl copper (B).
Explanation of Solution
n-Butyl bromide reacts with Li to yield n-butyl lithium and lithium bromide. The n-butyl lithium produced when reacted with CuI yields Lithium n-dibutyl copper as the product.
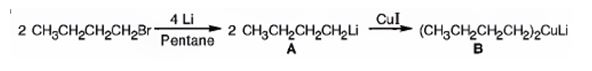
The products of the reaction shown are N-butyllithium (A) and Lithium n-dibutyl
g)
Interpretation:
The product of the reaction shown is to be predicted.
Concept introduction:
When alkyl halides are treated with lithium dimethyl copper, the halogens are displaced by an alkyl group and a higher alkane is produced as the product.
To predict:
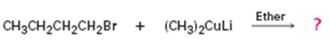
The product of the reaction in which n-butyl bromide reacts with lithium dimethylcopper.
Answer to Problem 26AP
The product of the reaction in which n-butyl bromide reacts with lithium dimethylcopper is n-pentane.
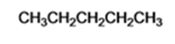
Explanation of Solution
When n-butyl bromide is treated with lithium dimethylcopper, the bromine is replaced by a methyl group to yield n-pentane as the product.
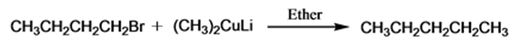
The product of the reaction in which n-butyl bromide reacts with lithium dimethylcopper is n-pentane.
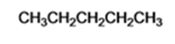
Want to see more full solutions like this?
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
- AN IR spectrum, a 13 CMR spectrum, and a 1 HMR spectrum were obtained for an unknown structure with a molecular formula of C9H10. Draw the structure of this compound.arrow_forwardAN IR spectrum, a 13 CMR spectrum, and a 1 HMR spectrum were obtained for an unknown structure with a molecular formula of C9H10. Draw the structure of this compound.arrow_forward(a) What is the hybridization of the carbon in the methyl cation (CH3*) and in the methyl anion (CH3¯)? (b) What is the approximate H-C-H bond angle in the methyl cation and in the methyl anion?arrow_forward
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor.arrow_forwardQ4: Draw the Lewis structures for the cyanate ion (OCN) and the fulminate ion (CNO). Draw all possible resonance structures for each. Determine which form for each is the major resonance contributor.arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. CH3 N CH3 HÖ: H3C CI: ::arrow_forward
- Q3: Draw the Lewis structures for nitromethane (CH3NO2) and methyl nitrite (CH3ONO). Draw at least two resonance forms for each. Determine which form for each is the major resonance contributor.arrow_forwardQ1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forwardQ2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forward
- Please correct answer and don't used hand raitingarrow_forward9. The following reaction, which proceeds via the SN1/E1 mechanisms, gives three alkene products (A, B, C) as well as an ether (D). (a) Show how each product arises mechanistically. (b) For the alkenes, determine the major product and justify your answer. (c) What clues in the reaction as shown suggest that this reaction does not go by the SN2/E2 mechanism route? (CH3)2CH-CH-CH3 CH3OH 1 Bl CH3OH ⑧· (CH3)2 CH-CH=CH2 heat H ⑥③ (CH3)2 C = C = CH3 © СнЗ-С-Снаснз сна (CH 3 ) 2 C H G H CH 3 оснзarrow_forwardPlease Don't used hand raitingarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

