
Applied Physics (11th Edition)
11th Edition
ISBN: 9780134159386
Author: Dale Ewen, Neill Schurter, Erik Gundersen
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.3, Problem 8P
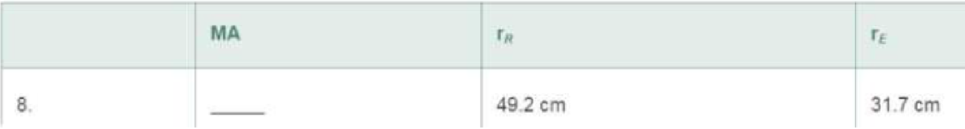
Given MAwheel-and-axle =
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
pls help on these
pls help on these
20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie
the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres
are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?
Chapter 10 Solutions
Applied Physics (11th Edition)
Ch. 10.2 - Given FRsR = FEsE,find eacd missing quantity.Ch. 10.2 - Given FRsR = FEsE,find eacd missing quantity.Ch. 10.2 - Given FRsR = FEsE,find eacd missing quantity.Ch. 10.2 - Given FRsR = FEsE,find eacd missing quantity.Ch. 10.2 - Given FRsR = FEsE,find eacd missing quantity.Ch. 10.2 - Given MAlever=FRFE,find each missing quantity.Ch. 10.2 - Given MAlever=FRFE,find each missing quantity.Ch. 10.2 - Given MAlever=FRFE,find each missing quantity.Ch. 10.2 - Given MAlever=FRFE,find each missing quantity.Ch. 10.2 - Given MAlever=FRFE,find each missing quantity.
Ch. 10.2 - Prob. 11PCh. 10.2 - Given MAlever = sEsR, find each missing quantity.Ch. 10.2 - Given MAlever = sEsR, find each missing quantity.Ch. 10.2 - Given MAlever = sEsR, find each missing quantity.Ch. 10.2 - A pole is used to lift a car that fell off a jack...Ch. 10.2 - Prob. 16PCh. 10.2 - A wheelbarrow 6.00 ft long is used to haul a...Ch. 10.2 - (a) Find the force, FE, pulling up on the beam...Ch. 10.3 - Given FRrR = FErE, find each missing quantity.Ch. 10.3 - Given FRrR = FErE, find each missing quantity.Ch. 10.3 - Given FRrR = FErE, find each missing quantity.Ch. 10.3 - Given FRrR = FErE, find each missing quantity.Ch. 10.3 - Given FRrR = FErE, find each missing quantity.Ch. 10.3 - Given MAwheel-and-axle = rErR, find each missing...Ch. 10.3 - Given MAwheel-and-axle.= rErR, find each missing...Ch. 10.3 - Given MAwheel-and-axle = rErR, find each missing...Ch. 10.3 - Given MAwheel-and-axle = rErR, find each missing...Ch. 10.3 - Given MAwheel-and-axle = rErR, find each missing...Ch. 10.3 - A wheel with radius 75.0 cm is attached to an axle...Ch. 10.3 - An axle of radius 12.0 cm is used with a wheel of...Ch. 10.3 - The radius of the axle of a winch is 3.00 in. The...Ch. 10.3 - A wheel of radius of 70.0 cm is attached to an...Ch. 10.3 - The diameter of the wheel of a wheel-and-axle is...Ch. 10.3 - Two persons use a large winch to raise a mass of...Ch. 10.4 - Find the mechanical advantage of each pulley...Ch. 10.4 - Prob. 2PCh. 10.4 - Prob. 3PCh. 10.4 - Find the mechanical advantage of each pulley...Ch. 10.4 - Find the mechanical advantage of each pulley...Ch. 10.4 - Prob. 6PCh. 10.4 - Prob. 7PCh. 10.4 - Prob. 8PCh. 10.4 - Draw each pulley system for Problems 914. 9. One...Ch. 10.4 - Two fixed and two movable with an MA of 5.Ch. 10.4 - Three fixed and three movable with an MA of 6.Ch. 10.4 - Four fixed and three movable. Find the systems MA.Ch. 10.4 - Four fixed and four movable with an MA of 8.Ch. 10.4 - Three fixed and four movable with an MA of 8.Ch. 10.4 - What is the MA of a single movable pulley?Ch. 10.4 - (a) What effort will lift a 250-lb weight by using...Ch. 10.4 - A system consisting of two fixed pulleys and two...Ch. 10.4 - A 400-lb weight is lifted 30.0 ft. (a) Using a...Ch. 10.4 - Can an effort force of 75.0 N lift a 275-N weight...Ch. 10.4 - (a) What effort will lift a 1950-N weight using...Ch. 10.4 - Can you arrange a pulley system containing 10...Ch. 10.5 - Given FRheight = FElength, find each missing...Ch. 10.5 - Given FRheight = FElength, find each missing...Ch. 10.5 - Given FRheight = FElength, find each missing...Ch. 10.5 - Given FRheight = FElength, find each missing...Ch. 10.5 - Given FRheight = FElength, find each missing...Ch. 10.5 - Given Mainclinedplane=lengthofplaneheightofplane,...Ch. 10.5 - Given Mainclinedplane=lengthofplaneheightofplane....Ch. 10.5 - Given Mainclinedplane=lengthofplaneheightofplane....Ch. 10.5 - Given Mainclinedplane=lengthofplaneheightofplane....Ch. 10.5 - Given Mainclinedplane=lengthofplaneheightofplane....Ch. 10.5 - Prob. 11PCh. 10.5 - A safe is loaded onto a truck whose bed is 5.50 ft...Ch. 10.5 - A 3.00-m-long plank is used to raise a cooling...Ch. 10.5 - A 2.75-m-long board is used to slide a compressor...Ch. 10.5 - Prob. 15PCh. 10.5 - A plank 12 ft long is used as an inclined plane to...Ch. 10.5 - Prob. 17PCh. 10.5 - A nursery loading dock is 1.20 m above the ground....Ch. 10.6 - Given FR pitch = FE 2r, find each missing...Ch. 10.6 - Given FR Pitch =FE 2r, find each missing...Ch. 10.6 - Given FR pitch = FE 2r, find each missing...Ch. 10.6 - Given FR pitch = FE 2r, find each missing...Ch. 10.6 - Given FR pitch = FE 2r, find each missing...Ch. 10.6 - Given MAscrew=2rpitch, find each missing quantity.Ch. 10.6 - Given MAscrew=2rpitch, find each missing quantity.Ch. 10.6 - Given MAscrew=2rpitch ,find each missing quantity.Ch. 10.6 - Given MAscrew=2rpitch , find each missing...Ch. 10.6 - Given MAscrew=2rpitch , find each missing...Ch. 10.6 - A 3650-lb car is raised using a jackscrew having...Ch. 10.6 - The mechanical advantage of a jackscrew is 97.0....Ch. 10.6 - A wood screw with pitch 0.125 in. is advanced into...Ch. 10.6 - The handle of a jackscrew is 60.0 cm long. (a) If...Ch. 10.8 - The box shown in Fig. 10.24 being pulled up an...Ch. 10.8 - The box shown in Fig. 10.24 being pulled up an...Ch. 10.8 - Find the mechanical advantage of the compound...Ch. 10.8 - If an effort of 300 lb is exerted, what weight can...Ch. 10.8 - What effort is required to move a load of 1.50...Ch. 10.8 - Find the mechanical advantage of the compound...Ch. 10.8 - What effort force is needed to move a box of...Ch. 10.8 - Find the mechanical advantage of the compound...Ch. 10.8 - If an effort of 450 N is exerted in Problem 8,...Ch. 10.8 - What effort force (in N) is needed to move 2.50...Ch. 10.9 - If the IMA of a ramp is 4.0 and its AMA is 3.2,...Ch. 10.9 - If the AMA of a wheel-and-axle is 9.0 and its IMA...Ch. 10.9 - If the efficiency of a screw is 32% and its IMA is...Ch. 10.9 - If the efficiency of lever is 94% and its AMA is...Ch. 10.9 - If the IMA of an inclined plane is 6.0 and its AMA...Ch. 10.9 - If the AMA of a ramp is 4.6 and its IMA is 6.0,...Ch. 10.9 - If the IMA of a pulley is 12 and its AMA is 9.0,...Ch. 10.9 - If the IMA of a screw is 60 and its AMA is 26,...Ch. 10.9 - If the efficiency of a pulley is 82% and its IMA...Ch. 10.9 - A wheel-and-axle has an efficiency of 65%. If its...Ch. 10 - Which of the following is not a simple machine? a....Ch. 10 - The force applied to the machine is the a. effort....Ch. 10 - Efficiency is a. the same as mechanical advantage....Ch. 10 - A second-class lever has a. two fulcrums. b. two...Ch. 10 - A pulley has eight strands holding the resistance....Ch. 10 - The mechanical advantage of a compound machine a....Ch. 10 - Cite three examples of machines used to multiply...Ch. 10 - What name is given to the force overcome by the...Ch. 10 - State the law of simple machines in your own...Ch. 10 - What is the term used for the ratio of the...Ch. 10 - What is the term used for the ratio of the amount...Ch. 10 - Does a frictionfree machine exist?Ch. 10 - What is the pivot point of a lever called?Ch. 10 - In your own words, state how to find the MA of a...Ch. 10 - Which type of lever do you think would be most...Ch. 10 - State the law of simple machines as it is applied...Ch. 10 - Where is the fulcrum located in a third-class...Ch. 10 - In your own words, explain the law of simple...Ch. 10 - Does the MA of a wheel-and-axle depend on the...Ch. 10 - Describe the difference between a fixed pulley and...Ch. 10 - Does the MA of a pulley depend on the radius of...Ch. 10 - How can you find the MA of an inclined plane?Ch. 10 - In your own words, describe the pitch of a screw.Ch. 10 - How does the MA of a jackscrew differ from the MA...Ch. 10 - A girl uses a lever to lift a box. The box has a...Ch. 10 - A bicycle requires 1575 N m of input but only puts...Ch. 10 - A lever uses an effort arm of 2.75 m and has a...Ch. 10 - Prob. 4RPCh. 10 - A wheel-and-axle has an effort force of 125 N and...Ch. 10 - What is the mechanical advantage of a pulley...Ch. 10 - A pulley system has a mechanical advantage of 5....Ch. 10 - An inclined plane has a height of 1.50 m and a...Ch. 10 - What height must a 10.0-ft-long inclined plane be...Ch. 10 - A screw has a pitch of 0.0200 cm. An effort force...Ch. 10 - A 945-N resistance force is overcome with a 13.5-N...Ch. 10 - Find the mechanical advantage of a jackscrew with...Ch. 10 - A courier uses a bicycle with rear wheel radius...Ch. 10 - (a) If the gear radius is doubled on the couriers...Ch. 10 - A farmer uses a pulley system to raise a 225-N...Ch. 10 - A laborer uses a lever to raise a 1250-N rock a...Ch. 10 - Prob. 17RPCh. 10 - Prob. 18RPCh. 10 - (a) Find the mechanical advantage of the compound...Ch. 10 - If an effort force of 45 N is applied to a simple...Ch. 10 - In the third century BC, Archimedes said, "Give me...Ch. 10 - Prob. 2ACCh. 10 - A snowblower auger has a radius of 7.75 in. and a...Ch. 10 - Aaron a bicycle mechanic, is studying the...Ch. 10 - Figure 10.32 5. Willie is using a wheelbarrow...
Additional Science Textbook Solutions
Find more solutions based on key concepts
67. We can make a static measurement to deduce the spring constant to use in the model. If a 61 kg woman stands...
College Physics: A Strategic Approach (3rd Edition)
SYNTHESIZE YOUR KNOWLEDGE Watennelon snow in Antarctica is caused by a species of photosynthetic green algae th...
Campbell Biology (11th Edition)
In what way do the membranes of a eukaryotic cell vary? A. Phospholipids are found only in certain membranes. B...
Campbell Biology in Focus (2nd Edition)
How Would the experiments result charge if oxygen (O2) were induced in the spark chamber?
Biology: Life on Earth with Physiology (11th Edition)
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
Why is petroleum jelly used in the hanging-drop procedure?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- pls help on all asked questions kindlyarrow_forward19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forwardpls help on allarrow_forward
- pls help on allarrow_forward6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forwardpls help on all asked questions kindlyarrow_forward
- pls help on all asked questions kindlyarrow_forward17. Two charges, one of charge +2.5 × 10-5 C and the other of charge +3.7 × 10-6 C, are 25.0 cm apart. The +2.5 × 10−5 C charge is to the left of the +3.7 × 10−6 C charge. a. Draw a diagram showing the point charges and label a point Y that is 20.0 cm to the left of the +3.7 × 10-6 C charge, on the line connecting the charges. (Field lines do not need to be drawn.) b. Calculate the net electric field at point Y.arrow_forward3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
What is Torque? | Physics | Extraclass.com; Author: Extraclass Official;https://www.youtube.com/watch?v=zXxrAJld9mo;License: Standard YouTube License, CC-BY