
Interpretation:
Major product has to be predicted for the given reaction.
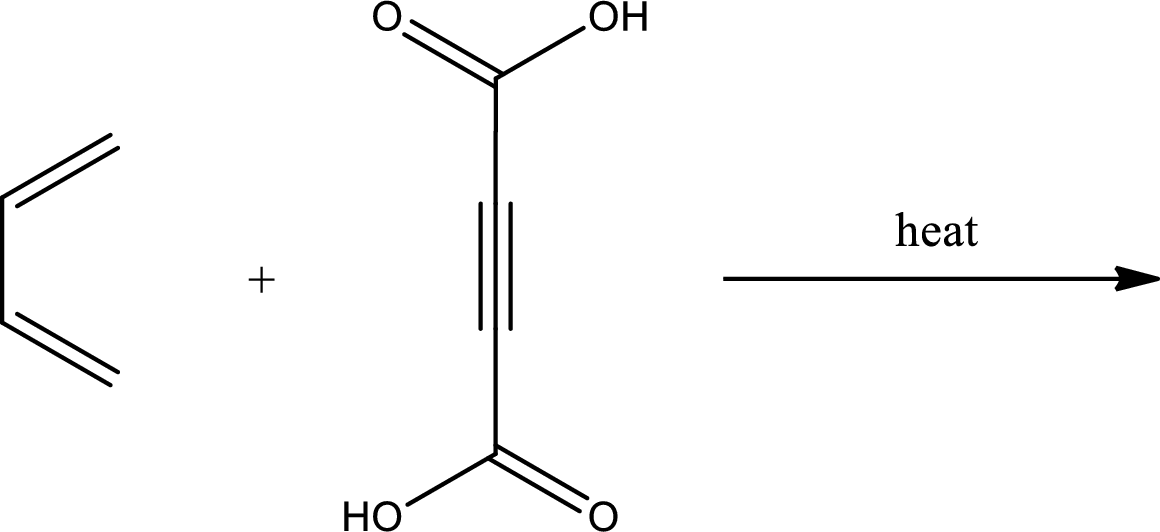
Concept Introduction:
Diels-Alder reaction is the 4+2 addition reaction. Here a total of six pi bonds are involved resulting in formation of one pi bond and two single bonds. Diels-Alder reaction occurs via a single concerted step and not by involvement of ions. If a monosubstituted ethylene is used, the product obtained contains a stereocenter. This can be represented as,
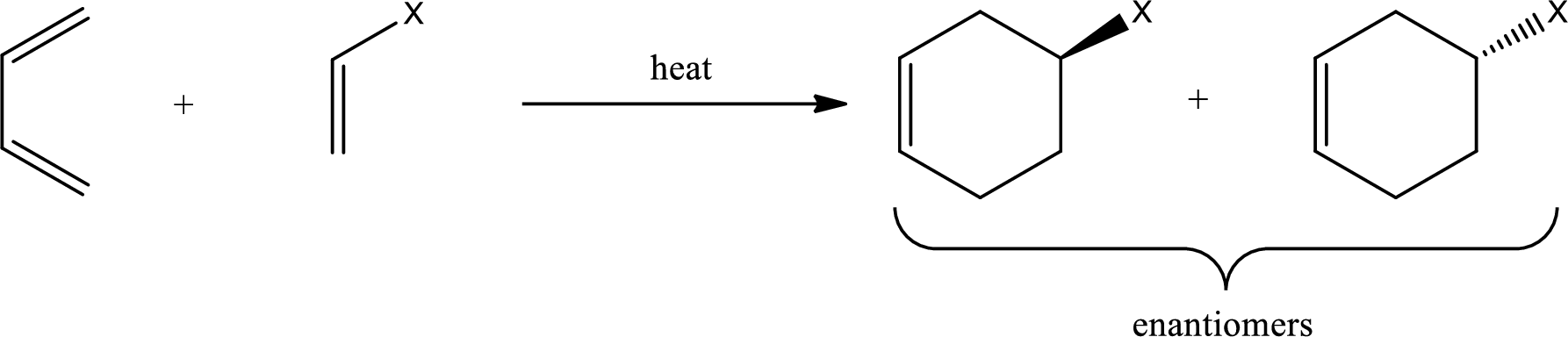
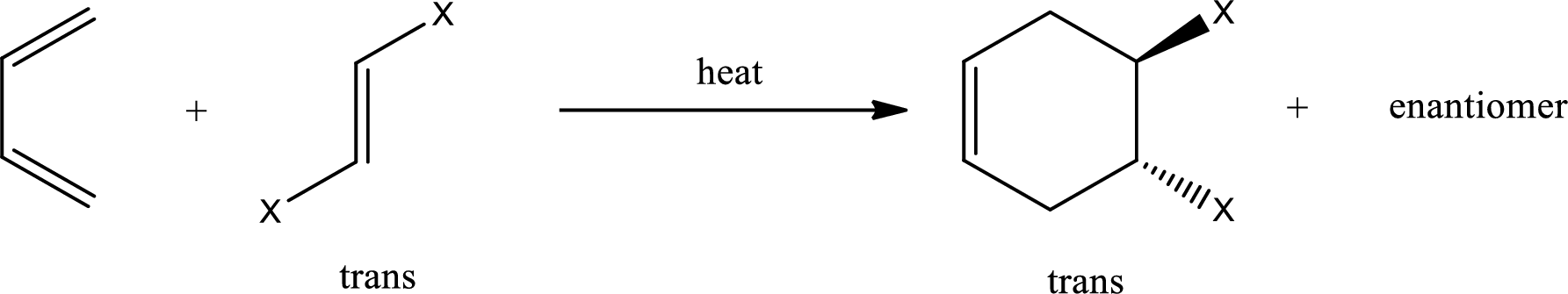
If 1,2-disubstituted ethylene is used as starting material, then the configuration of the dienophile is preserved in the product that is formed also. This means, if the dienophile is in cis-configuration, then the product formed will also have cis-configuration.

If the dienophile is in trans-configuration, then the product formed will also have trans-configuration.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

