
Organic Chemistry, Third Edition Binder Ready Version
3rd Edition
ISBN: 9781119110453
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10.10, Problem 22ATS
Interpretation Introduction
Interpretation:
Stereoisomers formed from the given hydrobromination reaction have to be given and also the relationship between those two isomers has to be given.
Concept Introduction:
Radical hydrobromination reaction:
Under photochemical condition
General mechanism for this reaction is illustrated below,
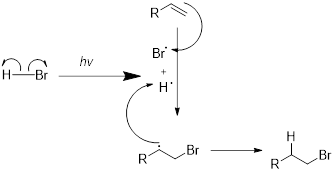
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Clouds of hot, luminous interstellar hydrogen gas can be seen in some parts of the galaxy. In some hydrogen atoms, electrons are excited to quantum levels with n = 100 or higher. (a) Calculate the wavelength observed on Earth if the electrons fall from the level with n = 100 to one with n = 2. (b) In what series would this transition be found? (c) Some of these high-energy electrons fall into intermediate states, such as n = 90. Would the wavelengths of a transition from the state with n = 100 to one with n = 90 be longer or shorter than those in the Balmer series? Explain your answer.
In the spectroscopic technique known as photoelectron spectroscopy (PES), ultraviolet radiation is directed at an atom or molecule. Electrons are ejected from the valence shell and their kinetic energies are measured. Since the energy of the incident ultraviolet photons is known and the kinetic energy of the ejected electron is measured, the ionization energy, I, can be deduced because total energy is conserved. (a) Show that the velocity, v, of the ejected electron and the frequency, n, of the incident radiation are related by hv = I + (1/2)mv^2? (b) Use this relation to calculate the ionization energy of a rubidium atom, knowing that light of wavelength 58.4 nm produces electrons with a velocity of 2,450 km/s Recall that 1 J = 1 kg.m^2/s^2
I) In Millikan's experiment, each droplet observed by the technicians contained an even number of electrons. If they had been unaware of this limitation, how would it have affected their report of an electron's charge?II) Millikan measured the charge of an electron in electrostatic units, esu. The data he collected included the following series of charges found on oil drops: 9.60 X 10^-10 esu, 1.92 X 10^-9 esu; 2.40 X 10^-9 esu; 2.88 X 10^-9 esu; and 4.80 X 10^-9 esu. (a) From this series, find the probable charge of the electron in electrostatic units. (b) Estimate the number of electrons in an oil drop with a charge of 6.72 X 10^-9 esu. The actual charge (in Coulombs) of an electron is 1.602 X 10^-19 C. What is the relationship between esu and Coulombs?
Chapter 10 Solutions
Organic Chemistry, Third Edition Binder Ready Version
Ch. 10.1 - Prob. 1CCCh. 10.1 - Prob. 1LTSCh. 10.1 - Prob. 2PTSCh. 10.1 - Prob. 3PTSCh. 10.1 - Prob. 4ATSCh. 10.1 - Prob. 2LTSCh. 10.1 - Prob. 5PTSCh. 10.1 - Prob. 6PTSCh. 10.1 - Prob. 7ATSCh. 10.2 - Prob. 3LTS
Ch. 10.2 - Prob. 8PTSCh. 10.2 - Prob. 9ATSCh. 10.3 - Prob. 4LTSCh. 10.3 - Prob. 10PTSCh. 10.3 - Prob. 11ATSCh. 10.5 - Prob. 5LTSCh. 10.5 - Prob. 12PTSCh. 10.5 - Prob. 13ATSCh. 10.6 - Prob. 6LTSCh. 10.6 - Prob. 14PTSCh. 10.6 - Prob. 15ATSCh. 10.7 - Prob. 7LTSCh. 10.7 - Prob. 16PTSCh. 10.7 - Prob. 17PTSCh. 10.7 - Prob. 18ATSCh. 10.8 - Prob. 19CCCh. 10.9 - Prob. 20CCCh. 10.10 - Prob. 8LTSCh. 10.10 - Prob. 21PTSCh. 10.10 - Prob. 22ATSCh. 10 - Prob. 23PPCh. 10 - Prob. 24PPCh. 10 - Prob. 25PPCh. 10 - Prob. 26PPCh. 10 - Prob. 27PPCh. 10 - Prob. 28PPCh. 10 - Prob. 29PPCh. 10 - Prob. 30PPCh. 10 - Prob. 31PPCh. 10 - Prob. 32PPCh. 10 - Prob. 33PPCh. 10 - Prob. 34PPCh. 10 - Prob. 35PPCh. 10 - Prob. 36PPCh. 10 - Prob. 37PPCh. 10 - Prob. 38PPCh. 10 - Prob. 39PPCh. 10 - Prob. 40PPCh. 10 - Prob. 41IPCh. 10 - Prob. 42IPCh. 10 - Prob. 43IPCh. 10 - Prob. 44IPCh. 10 - Prob. 45IPCh. 10 - Prob. 46IPCh. 10 - Prob. 47IPCh. 10 - Prob. 48IPCh. 10 - Prob. 49IPCh. 10 - Prob. 50IPCh. 10 - Prob. 51IPCh. 10 - Prob. 52IPCh. 10 - Prob. 53IPCh. 10 - Prob. 54IPCh. 10 - Prob. 55IPCh. 10 - Prob. 56IPCh. 10 - Prob. 57IPCh. 10 - Prob. 58CPCh. 10 - Prob. 59CPCh. 10 - As seen in this chapter, hydrocarbons typically do...Ch. 10 - Prob. 61CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- my ccc edu - Search X Quick Access X D2L Homepage - Spring 2025 x N Netflix X Dimensional Analysis - A x+ pp.aktiv.com Q ☆ X Question 59 of 70 The volume of 1 unit of plasma is 200.0 mL If the recommended dosage for adult patients is 10.0 mL per kg of body mass, how many units are needed for a patient with a body mass of 80.0 kg ? 80.0 kg 10.0 DAL 1 units X X 4.00 units 1 1 Jeg 200.0 DAL L 1 units X 200.0 mL = 4.00 units ADD FACTOR *( ) DELETE ANSWER RESET D 200.0 2.00 1.60 × 10³ 80.0 4.00 0.0400 0.250 10.0 8.00 & mL mL/kg kg units/mL L unit Q Search delete prt sc 111 110 19arrow_forwardIdentify the starting material in the following reaction. Click the "draw structure" button to launch the drawing utility. draw structure ... [1] 0 3 C10H18 [2] CH3SCH3 Harrow_forwardIn an equilibrium mixture of the formation of ammonia from nitrogen and hydrogen, it is found that PNH3 = 0.147 atm, PN2 = 1.41 atm and Pн2 = 6.00 atm. Evaluate Kp and Kc at 500 °C. 2 NH3 (g) N2 (g) + 3 H₂ (g) K₂ = (PN2)(PH2)³ = (1.41) (6.00)³ = 1.41 x 104arrow_forward
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forward
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY