
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.10, Problem 18P
Addition of
a. 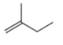 b.
b. 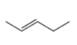 c.
c. 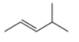
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Describe each highlighted bond in terms of the overlap of atomic orbitals.
(a)
Н
Н
H
H
[References]
HIC H
H C H
H-C-CC-N:
H
σ character n character
(b)
HIC H
H H
H-C-C-C
HIC H
Н
H
O-H
σ character
n character
Submit Answer
Try Another Version
3 item attempts remaining
11
Naming and drawing alcohols
Write the systematic (IUPAC) name for each of the following organic molecules:
structure
OH
HO
OH
Explanation Check
name
☐
what is the drawn mechanism for
diethyl carbonate and 4 - bromo - N, N
-dimethylaniline to create crystal
violet?
Chapter 10 Solutions
ORG CHEM CONNECT CARD
Ch. 10.1 - Prob. 1PCh. 10.2 - Problem 10.2 How many degrees of unsaturation are...Ch. 10.3 - Give the IUPAC name for each alkene. abcdeCh. 10.3 - Give the IUPAC name for each polyfunctional...Ch. 10.3 - Prob. 9PCh. 10.6 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10.7 - Prob. 12PCh. 10.9 - Problem 10.13 What product is formed when each...Ch. 10.9 - Prob. 14PCh. 10.10 - Problem 10.15 Draw the products formed when each...
Ch. 10.10 - Prob. 16PCh. 10.10 - Prob. 17PCh. 10.10 - Addition of HBr to which of the following alkenes...Ch. 10.11 - Problem 10.19 Draw the products, including...Ch. 10.11 - Prob. 20PCh. 10.12 - Problem 10.21 What two alkenes give rise to each...Ch. 10.12 - Prob. 22PCh. 10.13 - Problem 10.23 Draw the products of each reaction,...Ch. 10.14 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10.15 - Prob. 25PCh. 10.16 - Problem 10.26 What alkylborane is formed from...Ch. 10.16 - Draw the products formed when each alkene is...Ch. 10.16 - What alkene can be used to prepare each alcohol as...Ch. 10.16 - Prob. 29PCh. 10.17 - Draw the products of each reaction using the two...Ch. 10.18 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 43PCh. 10 - Prob. 44PCh. 10 - Prob. 45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 53PCh. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 60PCh. 10 - Prob. 61PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Give the IUPAC name for each compound.
Organic Chemistry
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following compounds are constitutional isomers of each other? I and II O II and III O III and IV OI and IV O II and IV CI H CI H CI H H CI H-C-C-CI C-C-C-CI H-C-C-CI H-C-C-CI H CI Ĥ ĆI A A Ĥ ĆI || IVarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardQ1: Curved Arrows, Bronsted Acids & Bases, Lewis Acids & Bases Considering the following reactions: a) Predict the products to complete the reactions. b) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw some of the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. c) Label Bronsted acids and bases in the left side of the reactions. Label conjugate acids and bases in the right side of the reactions. d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. + OH CH30: OH B. + HBr C. H₂SO4 D. CF 3. CH 3 + HCI N H fluoxetine antidepressant 1↓ JDownloadarrow_forward
- Don't used Ai solutionarrow_forwardPart 3: AHm,system Mass of 1.00 M HCI Vol. of 1.00 M HCI Mass of NaOH(s) Total Mass in Calorimeter Mole product if HCI limiting reactant Trial 1 62.4009 1.511g Mole product if NaOH limiting reactant Limiting reactant Initial Temperature Final Temperature 23.8°C 37.6°C Change in Temperature AHm,system (calculated) Average AHm,system (calculated) (calculated) (calculated) Trial 2 64.006g 1.9599 (calculated) (calculated) (calculated) (calculated) (calculated) (calculated) 24.7°C 41.9°C (calculated) (calculated) (2 pts. each)arrow_forwardDon't used Ai solutionarrow_forward
- What is the numerical value of the slope using the equation y=-1.823x -0.0162 please show calculationsarrow_forwardDon't used hand raitingarrow_forward1.) Using the graph below (including the line equation of y = -1.823x - 0.0162) What is the numerical value for the slope shown? 2.) What are the Unit(s) associated with the slope of the line shown? for we all remember that numerical data always has units. 3.) What would be a good title for this graph and explain your choice. 0.00 0.0 02 0.4 10.6 08 10 12 -0.20 -0.40 -0.60 -0.80 Temp, freezing, in degrees Celcius 5-1.00 -1.20 -1.40 -1:60 y=-1.823x-0.0162 -180 -2.00 Concentration of Sucrose (m)arrow_forward
- Don't used Ai solutionarrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling. Please label in the image, so it fits explanation. I am still very unsure I undertand this.arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License