
Concept explainers
Interpretation:
The major and minor products that are expected to be formed in the given reaction has to be identified.
Concept Introduction:
Three steps are followed for determining the products that will be formed in a
- 1. Function of reagent has to be determined.
- 2. The mechanism has to be determined by analyzing the substrate.
- 3. Relevant regiochemical and stereochemical requirements has to be considered.
Function of Reagent:
When a reagent functions as a nucleophile, substitution reaction takes place and when a reagent functions as a base, elimination reaction takes place. The first step is to determine the reagent to be strong or weak nucleophile and whether it is a strong or weak base. Basicity and nucleophilicity do not always parallel each other.
When comparing the atoms in the same row in periodic table, the basicity and nucleophilicity parallel each other. An example is,
When comparing the atoms in the same column in periodic table, the basicity and nucleophilicity do not parallel each other. An example is,
Basicity measures the charge stability on atom, while nucleophlicity measures how fast a nucleophile attacks. Basicity is a
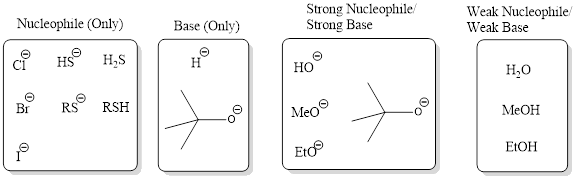
Nucleophile (Only): This category consists of reagents that act only as strong nucleophiles and not as bases. The reagent from this category involves in substitution reaction and not elimination.
Base (Only): This category consists of reagents that act only as bases and not as nucleophiles. The reagent from this category involves in elimination reaction and not substitution.
Strong Nucleophile/Strong Base: This category consists of reagents that are strong bases and also strong nucleophiles. This includes hydroxide, alkoxide ions. Generally these reagents are used for bimolecular process.
Weak Nucleophile/Weak Base: This category consists of reagents that are weak bases and weak nucleophile. This includes reagents such as water, alcohols. Generally these reagents are used for unimolecular process.
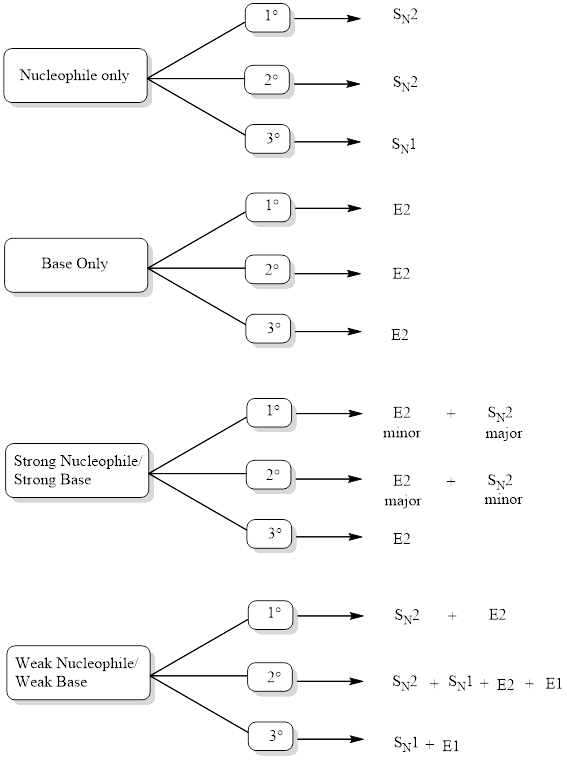
Determining Mechanism:
The mechanism can be identified by looking into the flowchart given below after analyzing the function of reagent.
Relevant regiochemical and stereochemical requirements:
| Mechanism | Regiochemical Outcome | Stereochemical Outcome |
| Attack of nucleophile takes place in the alpha position in which the leaving group is present | Nucleophile replaces the leaving group with the configuration inversion | |
| Nucleophile attacks carbocation. If rearrangement takes place, the carbocation will be different | Replacement of leaving group with racemization occurs | |
| E2 | Zaitsev product is favored over Hofmann product. | Process is stereospecific and stereoselective. |
| E1 | Always Zaitsev product is favored over Hofmann product. | Process is stereoselective. Trans substituted alkene is favored over cis substituted alkene. |
Answer to Problem 10.27P
The products that are obtained is,
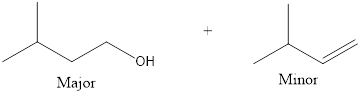
Explanation of Solution
Given reaction is,

Three steps are followed for determining the products that will be formed in a chemical reaction. They are,
- 1. Function of reagent has to be determined.
- 2. The mechanism has to be determined by analyzing the substrate.
- 3. Relevant regiochemical and stereochemical requirements has to be considered.
Analyzing the given reagent, sodium hydroxide is determined to be both strong nucleophile and strong base.
Looking into the mechanism, the substrate is a primary one. Hence,
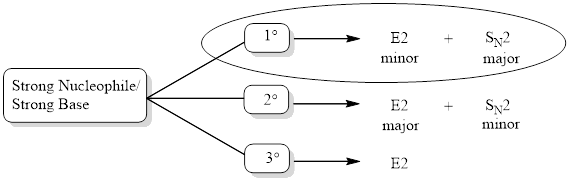
Looking at the regiochemical outcome, only one
The complete reaction can now be given as,
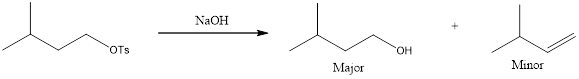
The expected products are identified for the given reaction.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





