
Concept explainers
(a)
Interpretation:
Lewis structure with the lowest formal charges of
Concept introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Formula to calculate the formal charge of the atom is as follows:
(a)
Answer to Problem 10.5BFP
Lewis structure with lowest formal charges for
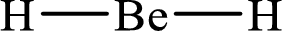
The formal charge on
The formal charge on each
Explanation of Solution
The total number of valence electrons of
Substitute 3 for the total number of
The Lewis structure of
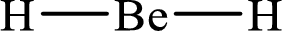
Substitute 2 for the number of valence electrons, 0 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 1 for the number of valence electrons, 0 for the number of nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each hydrogen atom.
(b)
Interpretation:
Lewis structure with the lowest formal charges of
Concept introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Formula to calculate the formal charge of the atom is as follows:
(b)
Answer to Problem 10.5BFP
The Lewis structure of
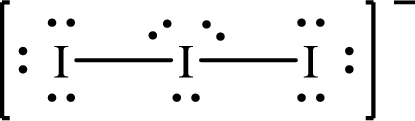
The formal charge on central
The formal charge on the terminal
Explanation of Solution
The total number of valence electrons of
Substitute 3 for the total number of
The Lewis structure of
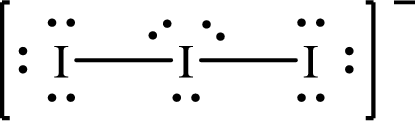
Substitute 7 for the number of valence electrons, 6 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on the central
Substitute 7 for the number of valence electrons, 6 for the number of nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each terminal
Only one Lewis structure is possible for
(c)
Interpretation:
Lewis structure with the lowest formal charges of
Concept introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Formula to calculate the formal charge of the atom is as follows:
(c)
Answer to Problem 10.5BFP
Lewis structure of
The formal charge on
The formal charge on each
Explanation of Solution
The Lewis structures of
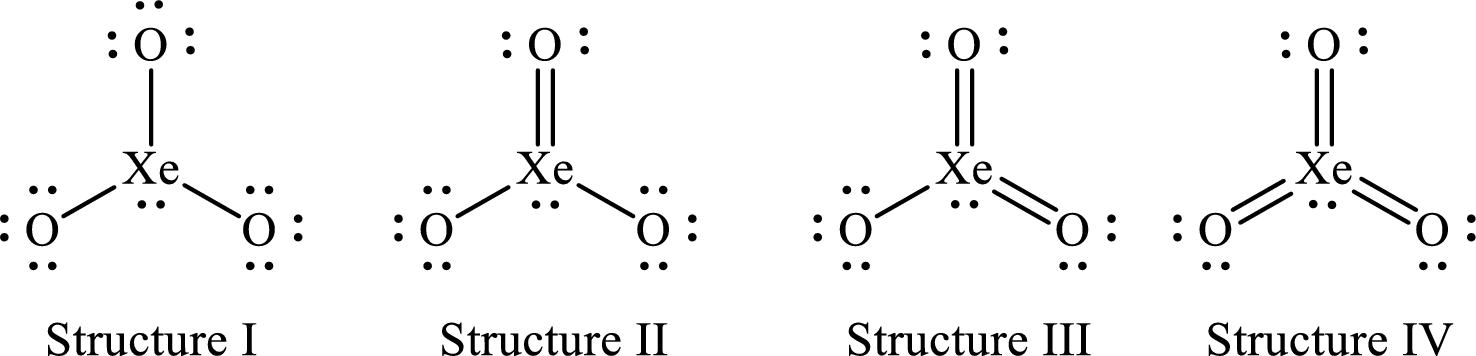
For structure I:
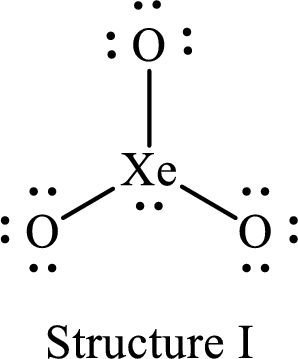
Substitute 8 for the number of valence electrons, 2 for the number of nonbonded electrons and 6 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for the number of valence electrons, 6 for the number of nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each single bonded oxygen atom.
For structure II:
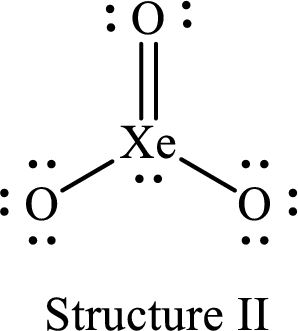
Substitute 8 for the number of valence electrons, 2 for the number of nonbonded electrons and 8 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for the number of valence electrons, 6 for the number of nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each single bonded oxygen atom.
Substitute 6 for the number of valence electrons, 4 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on the double bonded oxygen atom.
For structure III:
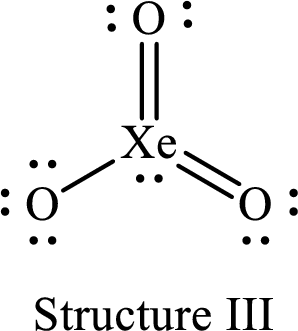
Substitute 8 for the number of valence electrons, 2 for the number of nonbonded electrons and 10 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for the number of valence electrons, 6 for the number of nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on the single bonded oxygen atom.
Substitute 6 for the number of valence electrons, 4 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on each double bonded oxygen atom.
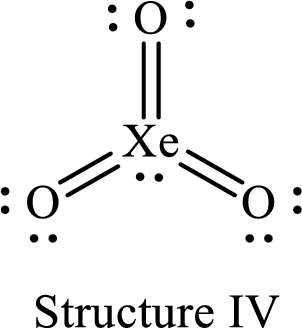
For structure IV:
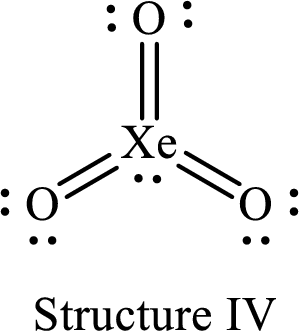
Substitute 8 for the number of valence electrons, 2 for the number of nonbonded electrons and 12 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for the number of valence electrons, 4 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on each double bonded oxygen atom.
Four Lewis structures are possible for
Want to see more full solutions like this?
Chapter 10 Solutions
Student Study Guide for Silberberg Chemistry: The Molecular Nature of Matter and Change
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
- In the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forwardCalculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





