
ORGANIC CHEMISTRY (LOOSELEAF)
6th Edition
ISBN: 9781260475630
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 64P
Draw a synthesis of each compound from cyclohexene as the starting material. More than one step is
needed.
a. 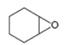 b.
b. 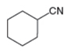 c.
c. 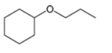 d.
d. 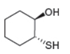
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q2: Label the following molecules as chiral or achiral, and label each stereocenter as R or S.
CI
CH3
CH3
NH2
C
CH3
CH3
Br
CH3
X
&p
Bra
'CH 3
"CH3
X
Br
CH3
Me - N
OMe
O
DuckDuck
1. For the four structures provided, Please answer the following questions in the table
below.
a. Please draw π molecular orbital diagram (use the polygon-and-circle method if
appropriate) and fill electrons in each molecular orbital
b. Please indicate the number of π electrons
c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non-
aromatic
TT MO diagram
Number of π e-
Aromaticity Evaluation (X choose one)
Non-aromatic
Aromatic
Anti-aromatic
||
|||
+
IV
1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?
Chapter 10 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)
Ch. 10.1 - Prob. 1PCh. 10.2 - Problem 10.2 How many degrees of unsaturation are...Ch. 10.3 - Give the IUPAC name for each alkene. abcdeCh. 10.3 - Give the IUPAC name for each polyfunctional...Ch. 10.3 - Prob. 9PCh. 10.6 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10.7 - Prob. 12PCh. 10.9 - Problem 10.13 What product is formed when each...Ch. 10.9 - Prob. 14PCh. 10.10 - Problem 10.15 Draw the products formed when each...
Ch. 10.10 - Prob. 16PCh. 10.10 - Prob. 17PCh. 10.10 - Addition of HBr to which of the following alkenes...Ch. 10.11 - Problem 10.19 Draw the products, including...Ch. 10.11 - Prob. 20PCh. 10.12 - Problem 10.21 What two alkenes give rise to each...Ch. 10.12 - Prob. 22PCh. 10.13 - Problem 10.23 Draw the products of each reaction,...Ch. 10.14 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10.15 - Prob. 25PCh. 10.16 - Problem 10.26 What alkylborane is formed from...Ch. 10.16 - Draw the products formed when each alkene is...Ch. 10.16 - What alkene can be used to prepare each alcohol as...Ch. 10.16 - Prob. 29PCh. 10.17 - Draw the products of each reaction using the two...Ch. 10.18 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 43PCh. 10 - Prob. 44PCh. 10 - Prob. 45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 53PCh. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 60PCh. 10 - Prob. 61PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
How does trandlation differ from transcription?
Microbiology: Principles and Explorations
Identify me theme or themes exemplified by (a) the sharp quills of a porcupine (b) the development of a multice...
Campbell Biology in Focus (2nd Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? H Br H Br (S) CH3 (R) CH3 H3C (S) H3C H Br Br H A C enantiomers H Br H Br (R) CH3 H3C (R) (S) CH3 H3C H Br Br H B D identicalarrow_forward2. Histamine (below structure) is a signal molecule involved in immune response and is a neurotransmitter. Histamine features imidazole ring which is an aromatic heterocycle. Please answer the following questions regarding Histamine. b a HN =N C NH2 a. Determine hybridization of each N atom (s, p, sp, sp², sp³, etc.) in histamine N-a hybridization: N-b hybridization: N-c hybridization: b. Determine what atomic orbitals (s, p, sp, sp², sp³, etc.) of the lone pair of each N atom resided in N-a hybridization: N-b hybridization: N-c hybridization:arrow_forwardNonearrow_forward
- 29. Use frontier orbital analysis (HOMO-LUMO interactions) to decide whether the following dimerization is 1) thermally allowed or forbidden and 2) photochemically allowed or forbidden. +arrow_forward30.0 mL of 0.10 mol/L iron sulfate and 20.0 mL of 0.05 mol/L of silver nitrate solutions are mixed together. Justify if any precipitate would formarrow_forwardDoes the carbonyl group first react with the ethylene glycol, in an intermolecular reaction, or with the end alcohol, in an intramolecular reaction, to form a hemiacetal? Why does it react with the alcohol it does first rather than the other one? Please do not use an AI answer.arrow_forward
- The number of noncyclic isomers that have the composition C4H8Owith the O as part of an OH group, counting a pair of stereoisomers as1, is A. 8; B. 6; C. 9; D. 5; E. None of the other answers is correct.arrow_forwardNonearrow_forwardThe number of carbon skeletons that have 8 carbons, one of which istertiary is A. 7; B. More than 7; C. 6; D. 5; E. 4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY