
Concept explainers
(a)
Interpretation:
1,2-dibromocyclohexane is an
(b)
Interpretation:
1,2-dibromocyclohexane can exist as cis- or trans- isomer has to be indicated as true or false and reason has to be described if it is false.
(c)
Interpretation:
1,2-dibromocyclohexane has lower boiling point than 1,2-dibromocycloheptane has to be indicated as true or false and reason has to be described if it is false.
(d)
Interpretation:
Given line formula of 1,2-dibromocyclohexane has to be indicated as true or false and reason has to be described if it is false.
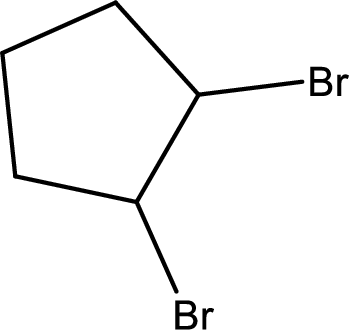
(e)
Interpretation:
1,2-dibromocyclohexane do not exist as multiple conformation has to be indicated as true or false and reason has to be described if it is false.
(f)
Interpretation:
One double bond is present in 1,2-dibromocyclohexane has to be indicated as true or false and reason has to be described if it is false.
(g)
Interpretation:
Reaction of 1,2-dibromocyclohexane with chlorine produces 1,2-dibromo-3-chlorocyclohexene has to be indicated as true or false and reason has to be described if it is false.
(h)
Interpretation:
1,2-dibromocyclohexane is a liquid at room temperature has to be indicated as true or false and reason has to be described if it is false.
(i)
Interpretation:
1,2-dibromocyclohexane substituted cycloalkane has to be indicated as true or false and reason has to be described if it is false.
(j)
Interpretation:
1,2-dibromocyclohexane is an
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
GENERAL, ORGANIC, BIOCHEM (LL W/ ACCESS)
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
- Please do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.arrow_forwardWhen two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forwardCalculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





