
Concept explainers
Give the IUPAC name for each compound.
a.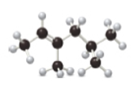 b.
b. 
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 32P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
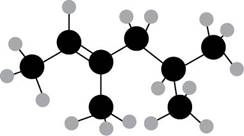
Figure 1
The ball and stick model of the compound is given. The black color ball represents the carbon atom and white color ball represent the hydrogen atom.
The numbering of carbon atoms present in the longest carbon chain is shown below.
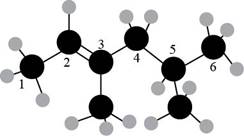
Figure 2
The longest carbon chain has six carbon atoms. The root word used for six carbon atoms is hex and the suffix used for
The IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 32P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
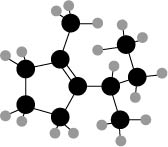
Figure 3
The ball and stick model of the compound is given. The black color ball represents the carbon atom and white color ball represent the hydrogen atom.
The numbering of carbon atoms present in the longest carbon chain is shown below.
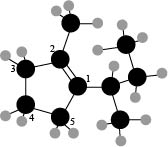
Figure 4
The longest carbon chain has five carbon atoms. The root word used for five carbon atoms is pent and the suffix used for
The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 10 Solutions
Connect Online Access 1-Semester for Organic Chemistry
Additional Science Textbook Solutions
Physics for Scientists and Engineers
Human Physiology: An Integrated Approach (8th Edition)
Anatomy & Physiology (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


