
Concept explainers
(a)
Interpretation:
Concept Introduction:
The atomic number of an atom is equal to the number of protons in it. The atomic number is represented by Z.
Answer to Problem 20P
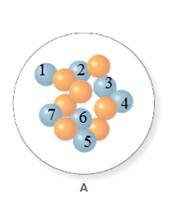
The atomic number for isotope of nitrogen A and B is 7.
Explanation of Solution
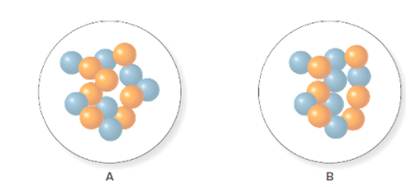
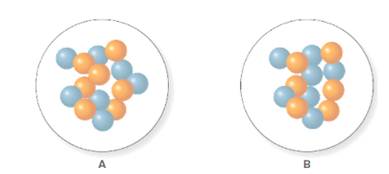
In the molecular model of fluorine, blue balls represent protons, whereas orange balls represent neutrons. Since the number of protons from given molecular model A and B is 7, the atomic number becomes 7 for A and B in both cases, as the atomic number is equal to the number of protons in that atom.
(b)
Interpretation:
The mass number for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
The mass number is equal to the number of protons and neutrons in an atom.
Answer to Problem 20P
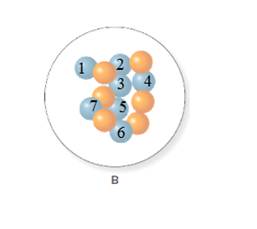
The mass number for isotope of nitrogen A and B is 14 and 13 respectively.
Explanation of Solution
From the molecular model of an isotope of nitrogen, the number of neutrons can be counted. Thus, the number of neutrons in A and B is 7 and 6 respectively.
As
For A and B, the mass number will be calculated as follows:
(c)
Interpretation:
The number of protons for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
The number of protons is equal to the atomic number of that atom.
Answer to Problem 20P
The number of protons in isotope of nitrogen A and B is 7.
Explanation of Solution
As the number of protons is equal to the atomic number in an atom. The atomic number of an isotope of nitrogen of A and B is 7. Thus, the number of protons in A and B will be 7.
(d)
Interpretation:
The number of neutrons for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
The mass number is the sum of all the protons and neutrons present in an atom. Thus, the number of neutrons can be calculated simply by subtracting the number of protons from the mass number of that atom.
Answer to Problem 20P
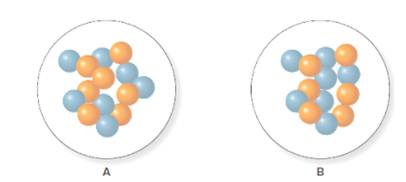
The number of neutrons in A and B is 7 and 6 respectively.
Explanation of Solution
For A the mass number and number of protons are 14 and 7 respectively. Thus, the number of neutrons in A will be calculated as follows:
For B the mass number and number of protons are 13 and 7 respectively. Thus, the number of neutrons in B will be calculated as follows:
(e)
Interpretation:
The isotope symbol for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
Isotopes are the compounds having the same atomic number but different
To write an isotope symbol atomic number (Z) is written on the lower left side and atomic mass(A) is written on the upper left side of an element.
Answer to Problem 20P
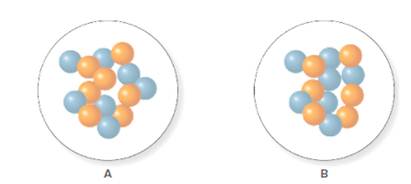
The isotope symbol for A and B is
Explanation of Solution
For A, the mass number and atomic number are 14 and 7 respectively. Thus, the isotope symbol for A will be represented as follows:
For B, the mass number and atomic number are 13 and 7 respectively. Thus, the isotope symbol for B will be represented as follows:
Want to see more full solutions like this?
Chapter 10 Solutions
General, Organic, and Biological Chemistry - 4th edition
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





