
Concept explainers
What are intermolecular forces? How do they differ from intramolecular forces? What are dipole-dipole forces? How do typical dipole-dipole forces differ from hydrogen bonding interactions? In what ways are they similar? What are London dispersion forces? How do typical London dispersion forces differ from dipole-dipole forces? In what ways are they similar? Describe the relationship between molecular size and strength of London dispersion forces. Place the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipole-dipole forces? Give an example of such an instance.
Interpretation:
- Intermolecular forces and its difference from intramolecular forces have to be described.
- Dipole-dipole forces and its variation and similarities with hydrogen bonding have to be described.
- London dispersion forces and its difference from dipole-dipole forces and similarities between them have to be explained.
- The relationship between molecular size and strength of London dispersion forces have to be described.
- Arrangement of major types of intermolecular forces in increasing order of strength has to be done.
- In case any deviation is present that has to be explained with an example.
Concept introduction:
Every atom strives to attain lowest possible energy in their shells. This is the driving force of atoms to combine with other atoms in so called “chemical reactions”. At the lowest possible energy levels, atoms and molecules attain utmost stability.
Reaching the lowest energy is not only the essential criterion for the molecules of matter to be stable. There are many other factors that have role in determining the stability of a substance. “Intermolecular forces” and “Intramolecular forces” are two such factors that have significant impact on the stability of matter.
In simple words, Intermolecular forces are termed as the forces acting “between molecules” that is components of a substance. Intramolecular forces are the forces that operate “within a molecule”. The prefix “inter” mean “among” and “intra” mean “within”.
Atoms do combine to form a molecule. Within a molecule, the atoms are held together by intramolecular forces. Many molecules are formed by such instance. Matter is composed of many such innumerable molecules which are held together by intermolecular forces. There are many types of intermolecular forces and intramolecular forces which is summarizes as follows –
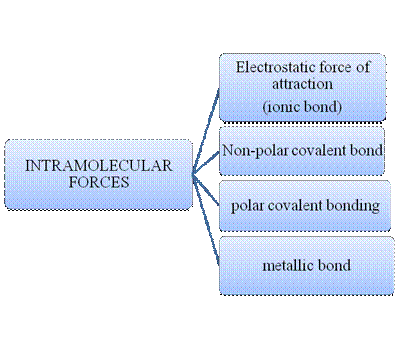
Figure 1
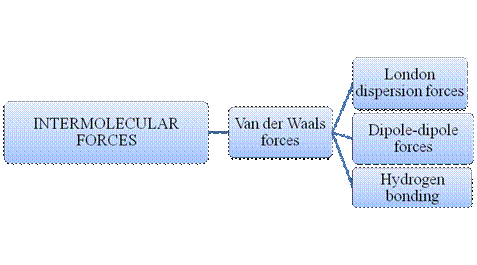
Figure 2
Intramolecular forces are nothing but the type of bonding between them. Ionic compounds have electrostatic force of attraction, the strongest one. Covalent bonds are of two types, that is polar and non-polar depend upon the polarity of the atoms. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
Answer to Problem 1RQ
Answer
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
- There exist no deviations in this arrangement.
Compare and contrast intermolecular forces and intramolecular forces.
Intermolecular forces are like cohesive forces, acting between the molecules whereas intramolecular forces are the forces exist within each molecule.
Explanation of Solution
Explanation
Intramolecular forces are responsible for the bond strength of a molecule. Stronger the intramolecular force stronger is the bond strength of a molecule. Stability at molecular level is highly significant.
The overall stability of a compound depends on how strong the molecules are held together. Intermolecular force is concerned about the overall stability of a substance. A stable substance has stronger intermolecular forces.
Intramolecular force is difficult to break than intermolecular force. For example, ionic bond or covalent is stronger than hydrogen bonding.
Despite, Intermolecular force is weaker than intramolecular force it has significance and impact on many of the properties of a substance.
Compare and contrast dipole-dipole forces and hydrogen bonding.
Dipole-dipole force and hydrogen bonding are the characteristic features of polar covalent compounds. Hydrogen bonding occurs only if the polar covalent compound has H-atom lies in close proximity to N, O or F whereas dipole-dipole forces exist in all polar compounds. Hydrogen bonding has high strength than dipole-dipole force.
A covalent bond is formed by mutual sharing of electrons between atoms. The two distinct types of covalent compounds are non-polar covalent and polar covalent compounds.
Atoms of the same element, particularly non-metals, bond which each other through covalent bond. There is no polarity between the atoms connected by the bond since the atoms have same electronegativity. Such type of compounds is non-polar covalent compounds. Hydrogen molecule is best example.
If atoms of slightly different electronegativity are covalently bonded, polarity arises spontaneously in the molecule due to the slight electronegativity difference between the atoms. Such compounds are polar covalent compounds. HCl molecule can be considered as example for this type.
In each molecule of a polar covalent compound, the electron cloud is displaced from the atom of low electronegativity to the atom of relatively high electronegativity through the covalent bond. As a result a “dipole” – a species containing weak partial positive and negative charge due to the unsymmetrical distribution of bonding electrons between atoms, is formed. Each dipole orient itself in such a direction that its positive end lies in close proximity to the negative end of the other dipole. The interaction between the dipoles is called “dipole-dipole forces”.
Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. This type of bonding is called hydrogen bonding and it is stronger than dipole-dipole forces. Hydrogen bonding has significant impact on stability, density and other properties of matter. Water is a best example of hydrogen bonding, in which each oxygen atom of a water molecule forms hydrogen bond with hydrogen of another water molecule.
Polarity is the main reason for the formation of both the forces. Dipole-dipole force is momentarily and has no significance when the molecules are not in close proximity. But hydrogen bonding is stronger than dipole-dipole forces. Hence, hydrogen bonding is a special type of dipole-dipole forces.
Compare and contrast London dispersion forces and dipole-dipole forces.
London dispersion forces exist in non-polar compounds whereas dipole-dipole forces exist in polar covalent compounds. Dipole-dipole force is stronger than London dispersion force.
Both polar and non-polar covalent compounds have London dispersion forces. These forces are due to temporary dipoles and do not exist permanently. The molecules convert to dipoles instantly and disappear. This is due to the uneven distribution of electrons between their atoms occurs momentarily when the bonded electrons approach nucleus. Thus it is a weakest force.
On the contrary, dipole-dipole force is applicable only to polar covalent compounds. Bonding between atoms of high electronegativity difference is ionic bond. But bonding between atoms of slightly varying electronegativity is polar covalent. Thus, the molecules here exist in the form of dipole due to the uneven distribution of electrons between their atoms. The interaction between the dipoles is dipole-dipole forces and it is relative stronger than London dispersion forces. Both of them exist temporarily.
Impact of molecular size on the strength of London dispersion forces.
Molecules having large size exert London dispersion forces to the large extent.
Larger size molecules have lesser interaction between nuclei and electrons. Thus the electrons are free from nuclear force of attraction and easily form dipoles. Thus, larger the size of the molecules, higher is the strength of London dispersion force.
Arrange the main types of intermolecular forces in increasing order of its strength.
Both London dispersion forces and dipole-dipole forces do not exist permanently. London dispersion force is due to temporary dipole whereas dipole-dipole force is due to temporary dipole and remains longer time than the former one. But hydrogen bonding exists permanently and thus it is the strongest among the intermolecular forces.
Analyze whether deviation is present in the above mentioned arrangement. If deviation is found justify it with suitable example.
No deviation is present in this arrangement.
Hydrogen bonding remains as the strongest intermolecular force always. There exists no deviation in the order arrangement of their respective strengths.
Conclusion
The differences between intermolecular forces and intramolecular forces have been described. Dipole–dipole forces and its variation with hydrogen bonding have been explained. London dispersion forces and its similarities and variation with dipole-dipole forces have been explained. The relationship between size of molecules and strength of London dispersion force has been explained. The major types of intermolecular forces are arranged in increasing order of its strength. No deviation is observed in the arrangement.
Want to see more full solutions like this?
Chapter 10 Solutions
Lab Manual for Zumdahl/Zumdahl/DeCoste¿s Chemistry, 10th Edition
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
- Answe Answer A and B pleasearrow_forward3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





