
Concept explainers
Interpretation:
The radicals, in order of theirs decreasing stability, are to be listed.
Concept introduction:
A molecule that contains at least one unpaired electron is known as a radical.
The relative stability of radicals is the same as that of carbocations.
The stability of radicals is as follows:
Answer to Problem 1PP
Solution:
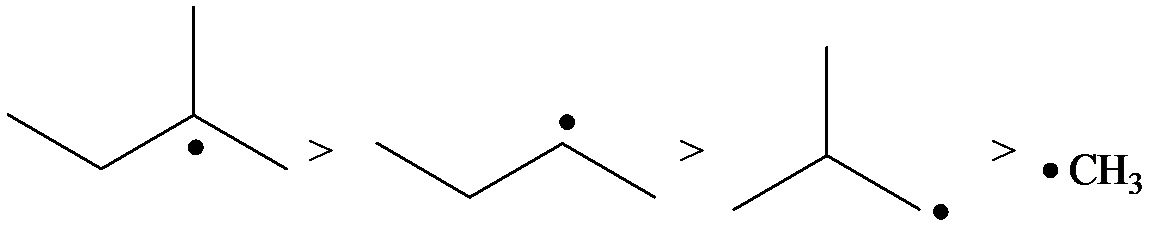
Explanation of Solution

The radical is a methyl radical. So, it is the least stable.
The radical is given as follows:
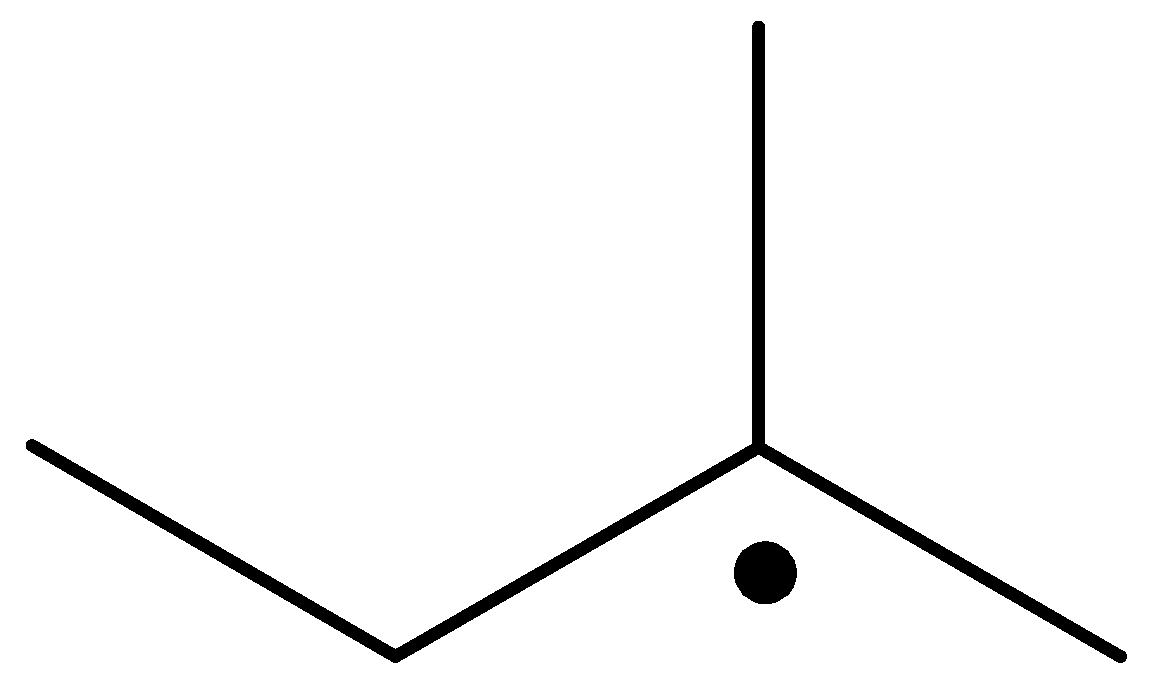
The carbon containing the unpaired electron is primary. Thus, the radical is a primary radical.
The radical is given as follows:
The carbon containing the unpaired electron is tertiary. Thus, the radical is a tertiary radical.
The radical is given as follows:
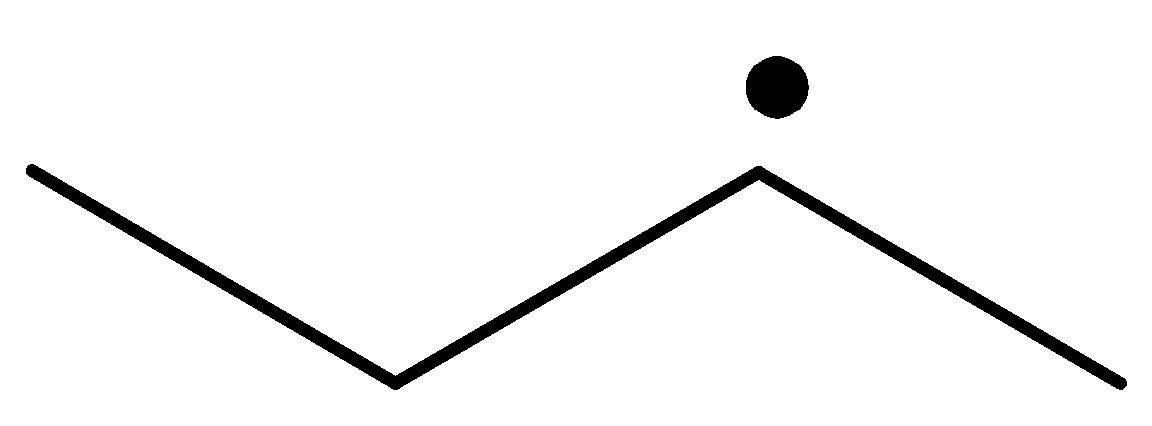
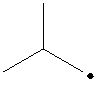
The carbon containing the unpaired electron is secondary. Thus, the radical is a secondary radical.
Therefore, the tertiary radical is the most stable and the methyl radical is the least stable.
Hence, the decreasing order of the stability of the radical is as follows:
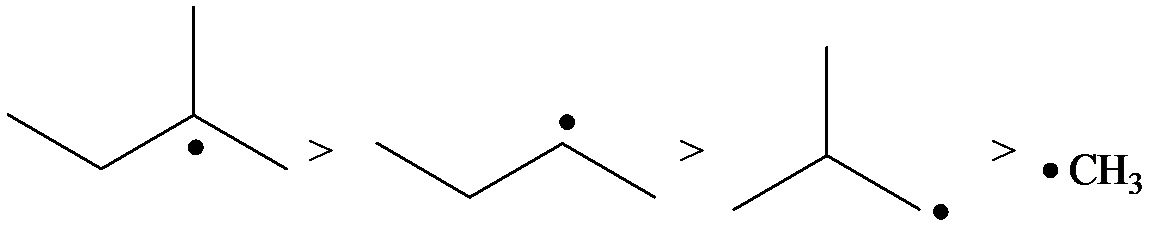
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
- Please help everysingle time ive asked in the past, the solution has been wrongarrow_forwardPlease helparrow_forward(a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

