
Concept explainers
(a)
Interpretation:
Pentane has higher melting point than decane has to be indicated as true or false.
(a)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. Decane contains ten carbon atoms and twenty two hydrogen atoms. As the molar mass increases, the London Dispersion forces become stronger and as a result melting and boiling points will also increase. Therefore, the given statement is false.
(b)
Interpretation:
Pentane is a substituted hydrocarbon has to be indicated as true or false.
(b)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

As seen from the above structure, there are no substituents present on the carbon chain. Hence, the given statement is a false one.
(c)
Interpretation:
Pentane is a saturated hydrocarbon has to be indicated as true or false.
(c)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

As there is only single bonds present between the carbon atoms in the entire molecule as shown above, pentane is a saturated hydrocarbon only. The given statement is true.
(d)
Interpretation:
Pentane is nonpolar has to be indicated as true or false.
(d)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

Pentane contains only carbon and hydrogen atom. Therefore, this is a nonpolar molecule only. Given statement is true.
(e)
Interpretation:
Pentane has structural formula of
(e)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. It is a linear
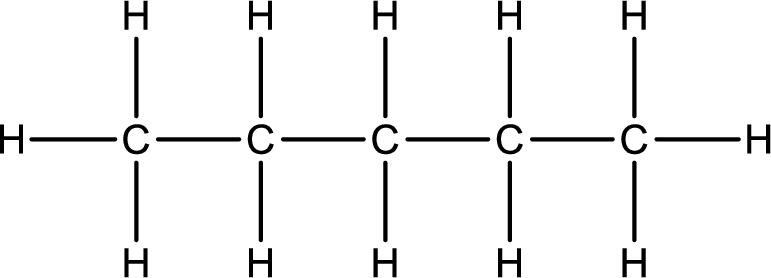
Molecular formula of pentane is only
(f)
Interpretation:
Pentane has weak London Dispersion forces than octane has to be indicated as true or false.
(f)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. Octane contains eight carbon atoms and eighteen hydrogen atoms. As the molar mass increases, the London Dispersion forces become stronger. This means pentane will have weaker London dispersion forces than octane. Hence, the given statement is true.
(g)
Interpretation:
Pentane is a gas at room temperature has to be indicated as true or false.
(g)
Explanation of Solution
Alkanes with one to four carbon atoms are gases at room temperature, and alkanes with five to seventeen carbon atoms are colorless liquids at room temperature. Pentane contains five carbon atoms in its skeleton. Hence, pentane is a liquid at room temperature. Therefore, the given statement is false.
(h)
Interpretation:
Pentane has only carbon‑carbon single bonds and carbon‑hydrogen single bonds has to be indicated as true or false.
(h)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

As there are no multiple bonds present in the above structure, pentane contains only single bonds between carbon and carbon, and carbon and hydrogen. Therefore, the given statement is true.
(i)
Interpretation:
Per mol of Pentane requires
(i)
Explanation of Solution
Pentane has a molecular formula as
Balanced chemical equation for the complete combustion of pentane can be given as,
As from the above equation it is understood that one mol of pentane requires eight mol of oxygen for complete combustion, the given statement is true.
(j)
Interpretation:
Pentane is a structural isomer of 2-methylpropane has to be indicated as true or false.
(j)
Explanation of Solution
Pentane has a molecular formula as
(k)
Interpretation:
Pentane on reaction with
(k)
Explanation of Solution
Pentane is a saturated hydrocarbon. It undergoes only substitution reaction and not addition reactions. Pentane does not undergo reaction with hydrogen bromide to form 1-bromopropane, 2-bromopropane, and 3-bromopropane. Therefore, the given statement is false.
Want to see more full solutions like this?
Chapter 10 Solutions
General, Organic, and Biochemistry
- 19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forwardQ4: Write organic product(s) of the following reactions and show the curved-arrow mechanism of the reactions. Br MeOH OSO2CH3 MeOHarrow_forward
- Provide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forwardQ6: Predict the major product(s) for the following reactions. Note the mechanism (SN1, SN2, E1 or E2) the reaction proceeds through. If no reaction takes place, indicate why. Pay attention to stereochemistry. NaCN DMF Br σ Ilm... Br H Br H H NaCN CH3OH KOtBu tBuOH NaBr H₂O LDA Et2O (CH3)2CHOH KCN DMSO NaOH H₂O, A LDA LDA Systemarrow_forwardQ7: For the following reactions, indicate the reaction conditions that would provide the indicated product in a high yield. Note the major reaction pathway that would take place (SN1, SN2, E1, or E2) Note: There may be other products that are not shown. There maybe more than one plausible pathway. Br H3C OH H3C CI ... H3C SCH2CH3 CI i SCH2CH3 ཨ་ Br System Settarrow_forward
- Q2: Rank the compounds in each of the following groups in order of decreasing rate of solvolysis in aqueous acetone. OSO2CF3 OSO2CH3 OH a. b. CI Brarrow_forwardох 4-tert-butyl oxy cyclohex-1-ene Incorrect, 1 attempt remaining The systematic name of this compound classifies the -OR group as a substituent of the hydrocarbon, which is considered the principal functional group. The ether substituent is named with the suffix 'oxy'. The general format for the systematic name of a hydrocarbon is: [prefix/substituent] + [parent] + [functional group suffix] Substituents are listed in alphabetical order. Molecules with a chiral center will indicate the absolute configuration at the beginning of its name with the R and S notation.arrow_forward5. Compressibility (6 points total). The isothermal compressibility is a measure of how hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is др defined as Br=-()=-(200²)T' (a) You might wonder why there is a negative sign in this formula. What does it mean when this quantity is positive and what does it mean when this quantity is negative? (b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!) (c) Explain under what conditions for the ideal gas the compressibility is higher or lower, and why that makes sense.arrow_forward
- 19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more reactive in this reaction? Briefly explain your choice using structures to support your answer. Mere-eries-cecleone) The tran-i-browse-3-methylcyclohexionearrow_forwardPlease help me calculate the undiluted samples ppm concentration. My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve. Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4arrow_forwardProvide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) H₁₂C C(CH3)3 C=C H3C CH3 CH3CH2CH CI CH3 Submit Answer Retry Entire Group 2 more group attempts remaining Previous Nextarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





