
Concept explainers
Write Lewis symbols for the following atoms. (a) Kr; (b) Ge; (c) N; (d) Ga; (e) As; (f) Rb.
(a)
Interpretation:
Interpret Lewis symbol of Kr.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Kr is:
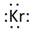
Explanation of Solution
In periodic table, Krypton is present in 18 group and in 4 period. The symbol of Krypton is Kr carrying 8 valence electrons in its valence shell.
Thus, the Lewis symbol of Kr is:
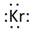
(b)
Interpretation:
Interpret the Lewis symbol of Ge.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Ge is:

Explanation of Solution
In periodic table, Germanium is present in 14 group and in 4 period. The symbol of Germanium is Ge carrying 4 valence electrons in its valence shell.
Thus, the Lewis symbol of Ge is:

(c)
Interpretation:
Interpret the Lewis symbol of N.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of N is:
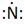
Explanation of Solution
In periodic table, Nitrogen is present in 15 group and in 2 period. The symbol of Nitrogen is N carrying 5 valence electrons in its valence shell.
Thus, the Lewis symbol of N is:
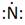
(d)
Interpretation:
Interpret the Lewis symbol of Ga.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Ga is:
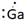
Explanation of Solution
In periodic table, Gallium is present in 13 group and in 4 period. The symbol of Gallium is Ga carrying 3 valence electrons in its valence shell.
Thus, Lewis symbol of Ga is:
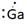
(e)
Interpretation:
Interpret the Lewis symbol of As.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of As is:
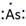
Explanation of Solution
In periodic table, Arsenic is present in 15 group and in 4 period. The symbol of Arsenic is As carrying 5 valence electrons in its valence shell.
Thus, the Lewis symbol of As is:
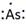
(f)
Interpretation:
Interpret the Lewis symbol of Rb.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Rb is:
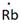
Explanation of Solution
In periodic table, Rubidium is present in 1 group and in 5th period. The symbol of rubidium is Rb carrying 1 valence electron in its valence shell.
Thus, the Lewis symbol of Rb is:
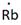
Want to see more full solutions like this?
Chapter 10 Solutions
EBK GENERAL CHEMISTRY
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (6th Edition)
Campbell Biology (11th Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Nonearrow_forward4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward
- 3. 2. 1. On the graph below, plot the volume of rain in milliliters versus its height in centimeters for the 400 mL beaker. Draw a straight line through the points and label it "400 mL beaker." Volume (mL) 400 350 300 250 200 150 750 mL Florence Volume Versus Height of Water 400 mL beaker 100 50 0 0 2 3 4 5 Height (cm) 6 7 8 9 10 Explain why the data points for the beaker lie roughly on a straight line. What kind of relationship is this? How do you know? (see page 276 text) the design of the beaker is a uniform cylinder the volume of liquid increases evenly with its height resulting in a linear relationship. What volume would you predict for 10.0 cm of water? Explain how you arrived at your answer. Use the data table and the graph to assist you in answering the question. 4. Plot the volume of rain in milliliters versus its height in centimeters for the 250 mL Florence flask on the same graph. Draw a best-fit curve through the points and label it "250 mL Florence flask." oke camearrow_forwardShow work. Don't give Ai generated solutionarrow_forwardIn the video, we looked at the absorbance of a certain substance and how it varies depending on what wavelength of light we are looking at. Below is a similar scan of a different substance. What color BEST describes how this substance will appear? Absorbance (AU) Violet Blue Green Orange 1.2 1.0- 0.8- 0.6- 0.4- 0.2 0.0 450 500 550 600 650 700 Wavelength (nm) violet indigo blue green yellow orange red Red O Cannot tell from this information In the above graph, what causes -450 nm wavelength of light to have a higher absorbance than light with a -550 nm wavelength? Check all that are true. The distance the light travels is different The different data points are for different substances The concentration is different at different times in the experiment Epsilon (molar absortivity) is different at different wavelengthsarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





