
Concept explainers
(a)
Interpretation:
The given molecular shapes are to be matched with their corresponding species.
Concept introduction:
VSEPR theory is based on the repulsions between the electrons pair in the valence shell. According to VSEPR theory, the shape of a molecule depends on the position of the electron pairs to minimize the repulsion, and maximize the stability.
According to VSEPR theory, the table for molecular geometries when the central atom has no lone pair is as follows:
(b)
Interpretation:
The polar molecular shape is to be identified.
Concept introduction:
Dipole moment arises when there is a separation of charges between two ions or atoms involved in the bond. The dipole moment is a vector quantity and its direction towards the most electronegative atom.
The direction of the dipole moment is represented as follows:
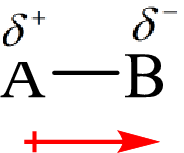
The polar and non-polar molecule can be identified on the basis of the net dipole moment. Polar molecules have non zero value of net dipole moment and the nonpolar molecules have zero net dipole moment.
(c)
Interpretation:
Molecular shape with the most valence electron around the central atom is to be identified.
Concept introduction:
Valence electrons are the outermost electrons of the central atom in its valence shell. For example, oxygen has
Trending nowThis is a popular solution!

Chapter 10 Solutions
CHEMISTRY:MOLEC NAT PRINT COMPANION
- The reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardWhat is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forward
- Provide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forwardProvide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forwardQuestion 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





