
Concept explainers
Predict the bond angles for the following molecules: (a) BeCl2, (b) BCl3, (c) CCl4, (d) CH3Cl, (e) Hg2Cl2 (arrangement of atoms: ClHgHgCl), (f) SnCl2, (g) H2O2, (h) SnH4.
(a)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 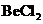 is
is 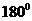 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
 .
.
Lewis structure of the given molecule is drawn below.
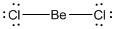
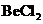 is a triatomic molecule . Here the central atom beryllium atom does not have any lone pair of electrons whereas both terminal chlorine atoms have 3 pairs of electron. It is a
is a triatomic molecule . Here the central atom beryllium atom does not have any lone pair of electrons whereas both terminal chlorine atoms have 3 pairs of electron. It is a 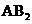 type molecule. Since there are only two bonds, there is only one bond angle. Since there is no lone pair on the central atom, to minimize the repulsion, they form a linear geometry. So the bond angle between two atoms is
type molecule. Since there are only two bonds, there is only one bond angle. Since there is no lone pair on the central atom, to minimize the repulsion, they form a linear geometry. So the bond angle between two atoms is 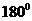 .
.
(b)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 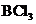 is
is 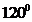 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
 .
.
Lewis structure of the given molecule is drawn below.
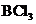 contains four atoms. Here the central atom boron atom does not have any lone pair of electrons whereas the terminal chlorine atoms have 3 pairs of electron. It is a
contains four atoms. Here the central atom boron atom does not have any lone pair of electrons whereas the terminal chlorine atoms have 3 pairs of electron. It is a 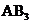 type molecule. Since there are only three bonds, there are two bond angle. Since there is no lone pair on the central atom, to minimize the repulsion, they form a trigonal planar geometry. So the bond angle between two atoms is
type molecule. Since there are only three bonds, there are two bond angle. Since there is no lone pair on the central atom, to minimize the repulsion, they form a trigonal planar geometry. So the bond angle between two atoms is 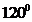 .
.
(c)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 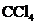 is
is 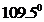 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
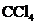 .
.
Lewis structure of the given molecule is drawn below.
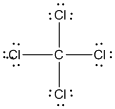
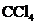 contains five atoms. Here the central atom carbon atom does not have any lone pair of electrons whereas the terminal chlorine atoms have 3 pairs of electron. It is a
contains five atoms. Here the central atom carbon atom does not have any lone pair of electrons whereas the terminal chlorine atoms have 3 pairs of electron. It is a 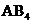 type molecule. Since there is no lone pair on the central atom, to minimize the repulsion, they form a tetrahedral geometry. So the bond angle between two atoms is
type molecule. Since there is no lone pair on the central atom, to minimize the repulsion, they form a tetrahedral geometry. So the bond angle between two atoms is 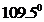 .
.
(d)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 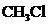 is
is 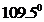 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
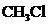 .
.
Lewis structure of the given molecule is drawn below.
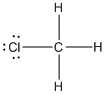
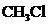 contains five atoms. Here the central atom carbon atom does not have any lone pair of electrons whereas the terminal chlorine atom has 3 pairs of electron. It is a
contains five atoms. Here the central atom carbon atom does not have any lone pair of electrons whereas the terminal chlorine atom has 3 pairs of electron. It is a 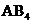 type molecule. Since there is no lone pair on the central atom, to minimize the repulsion, they form a distorted tetrahedral geometry because of the size difference of terminal chlorine and hydrogen atoms. So the bond angle between two atoms is
type molecule. Since there is no lone pair on the central atom, to minimize the repulsion, they form a distorted tetrahedral geometry because of the size difference of terminal chlorine and hydrogen atoms. So the bond angle between two atoms is 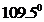 .
.
(e)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 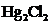 is
is 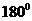 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
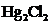 .
.
Lewis structure of the given molecule is drawn below.
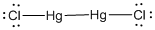
In the case of 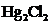 , both mercury atom does not have any lone pair of electrons whereas the terminal chlorine atoms have 3 pairs of electron. Both the mercury atom is of
, both mercury atom does not have any lone pair of electrons whereas the terminal chlorine atoms have 3 pairs of electron. Both the mercury atom is of 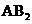 type molecule. . Since there is no lone pair on the central atom, to minimize the repulsion, they form a linear geometry. So the bond angle between two atoms is
type molecule. . Since there is no lone pair on the central atom, to minimize the repulsion, they form a linear geometry. So the bond angle between two atoms is 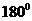 .
.
(f)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of  is
is 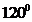 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
 .
.
Lewis structure of the given molecule is drawn below.
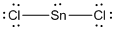
In the case of  , the central atom tin atom have a lone pair of electron whereas the terminal chlorine atoms have 3 pairs of electron and is a
, the central atom tin atom have a lone pair of electron whereas the terminal chlorine atoms have 3 pairs of electron and is a 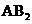 with one lone pair of electron type molecule. There are only two bonds so there is only one bond angle. Since there is one lone pair on the central atom, to minimize the repulsion, they form a bent geometry. So the bond angle between two atoms is
with one lone pair of electron type molecule. There are only two bonds so there is only one bond angle. Since there is one lone pair on the central atom, to minimize the repulsion, they form a bent geometry. So the bond angle between two atoms is 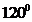 .
.
(g)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 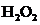 is
is 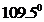 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
 .
.
Lewis structure of the given molecule is drawn below.
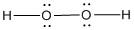
In the case of  , both oxygen atom have any two lone pair of electrons and is a
, both oxygen atom have any two lone pair of electrons and is a 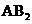 with two lone pair of electron type molecule.. Since there is two lone pair on each oxygen atom, to minimize the repulsion, they form a tetrahedral geometry. So the bond angle between two atoms is
with two lone pair of electron type molecule.. Since there is two lone pair on each oxygen atom, to minimize the repulsion, they form a tetrahedral geometry. So the bond angle between two atoms is 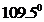 .
.
(h)
Interpretation: The bond angle of the given molecule should be found.
Concept Introduction:
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Answer to Problem 10.80QP
The bond angle of 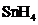 is
is 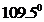 .
.
Explanation of Solution
To find: The bond angle of the given molecule
Given molecule is
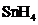 .
.
Lewis structure of the given molecule is drawn below.
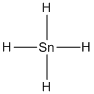
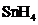 Contains five atoms. Here the central atom tin atom does not have any lone pair of electrons. It is a
Contains five atoms. Here the central atom tin atom does not have any lone pair of electrons. It is a 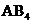 type molecule. Since there is no lone pair on the central atom, to minimize the repulsion, they form a tetrahedral geometry. So the bond angle between two atoms is
type molecule. Since there is no lone pair on the central atom, to minimize the repulsion, they form a tetrahedral geometry. So the bond angle between two atoms is 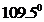 .
.
Want to see more full solutions like this?
Chapter 10 Solutions
CHEMISTRY (LL) W/CNCT >BI<
- Sketch the intermediates for A,B,C & D.arrow_forwardCan the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? O ? A . If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. ㅇ 80 F5 F6 A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente FIGarrow_forwardIn methyl orange preparation, if the reaction started with 0.5 mole of sulfanilic acid to form the diazonium salt of this compound and then it converted to methyl orange [0.2 mole]. If the efficiency of the second step was 50%, Calculate: A. Equation(s) of Methyl Orange synthesis: Diazotization and coupling reactions. B. How much diazonium salt was formed in this reaction? C. The efficiency percentage of the diazotization reaction D. Efficiency percentage of the whole reaction.arrow_forward
- Hand written equations pleasearrow_forwardHand written equations pleasearrow_forward> each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X Ś CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) © 2025 McGraw Hill LLC. All Rights Farrow_forward
- NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forwardHow many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forward
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





