
Concept explainers
Interpretation: The bond moments and the resultant dipole moments of the given molecules have to be sketched out.
Concept Introduction:
Dipole moment:
The term dipole moment refers to the quantitative measure of polarity of a bond. It is represented as
For example:
The diatomic molecule
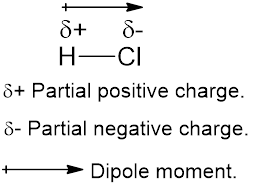
The crossed arrow represents the direction of the shift of electrons toward the highly electronegative chlorine atom from the least electronegative hydrogen atom.
Bond moment:
In polar molecules, there will be a bond between a high electronegative atom and a least electronegative atom. The high electronegative atom pulls the shared pair of electrons towards itself and acquires a partial negative charge on it and creates a partial positive charge on the least electronegative atom. This is known as charge separation and the overall phenomenon is known as bond moment. It is a vector quantity which means it has both magnitude and direction.
Resultant dipole moment:
If all possible bond moments of a polar molecule are with different magnitudes, then a net dipole moment will be resulted which is known as the resultant dipole moment. If the bond moments are with same magnitudes, then there won’t be a resultant dipole moment since all the bond moments cancel with each other. So, the resultant dipole moment depends on the magnitude of bond moments. It is independent of the directions of bond moments.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
- What is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward> aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forwardThere are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forward
- EEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forwardAnswerarrow_forward2. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. CH3 Ph OEt هد Ph CH3 Hint: the species on the left is an ynolate, which behaves a lot like an enolate.arrow_forward
- b. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forwardb. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forwardPredict the major products of this reaction: excess H+ NaOH ? A Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second. If there won't be any products, just check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privarrow_forward
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





