
Concept explainers
(a)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
The dipole moment arises when there is a separation of charges between two ions or atoms involved in the bond. The dipole moment is a vector quantity and its direction towards the most electronegative atom.
The direction of the dipole moment is represented as follows:
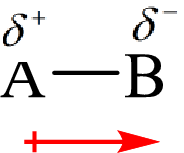
The polar and non-polar molecule can be identified on the basis of the net dipole moment. Polar molecules have non zero value of net dipole moment and the non polar molecules have zero net dipole moment.
(a)
Answer to Problem 10.57P
Explanation of Solution
The shape of
The dipole moment in
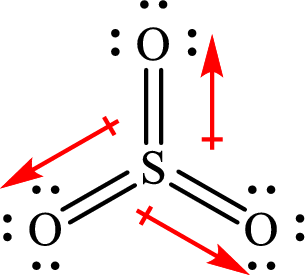
All the
The shape of
The dipole moment in
The individual dipole moment does not cancel each other so the value of net dipole moment is not zero and therefore
Hence,
Molecules in which individual dipole moment does not cancel each other result in net non-zero dipole moment and the overall molecule is said to be polar. In
(b)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge.
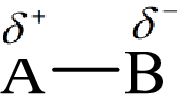
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
The greater electronegativity difference between the atoms induces the greater polarity in the bond thus it generates higher dipole moment.
(b)
Answer to Problem 10.57P
Explanation of Solution
The formula to calculate
Substitute
The formula to calculate
Substitute
Bond polarity and dipole moment are directly related to the electronegativity difference. Therefore,
Greater the electronegativity difference between the two bonding atoms more polar will be the bond.
(c)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
The dipole moment arises when there is a separation of charges between two ions or atoms involved in the bond. The dipole moment is a vector quantity and its direction towards the most electronegative atom.
The direction of the dipole moment is represented as follows:
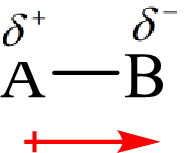
The polar and non-polar molecule can be identified on the basis of the net dipole moment. Polar molecules have non zero value of net dipole moment and the non polar molecules have zero net dipole moment.
(c)
Answer to Problem 10.57P
Explanation of Solution
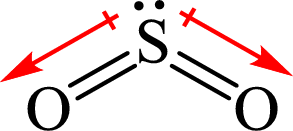
The shape of
The dipole moment in
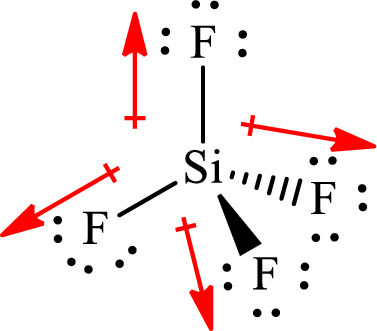
All the
The shape of
The dipole moment in
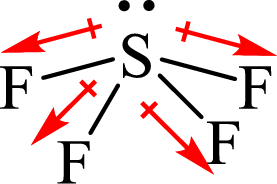
The individual dipole moment does not cancel each other so the value of net dipole moment is not zero and therefore
Hence,
Molecules in which individual dipole moment does not cancel each other result in net non-zero dipole moment and the overall molecule is said to be polar. In
(d)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge.
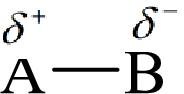
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
The greater electronegativity difference between the atoms induces the greater polarity in the bond thus it generates higher dipole moment.
(d)
Answer to Problem 10.57P
Explanation of Solution
The formula to calculate
Substitute
The formula to calculate
Substitute
Both
Greater the electronegativity difference between the two bonding atoms more polar will be the bond.
Want to see more full solutions like this?
Chapter 10 Solutions
MCGRAW: CHEMISTRY THE MOLECULAR NATURE
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





