
Bundle: General Chemistry, Loose-Leaf Version, 11th + LabSkills PreLabs v2 for Organic Chemistry (powered by OWLv2), 4 terms (24 months) Printed ... for Ebbing/Gammon's General Chemistry, 11th
11th Edition
ISBN: 9781337542630
Author: Darrell Ebbing, Steven D. Gammon
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.48QP
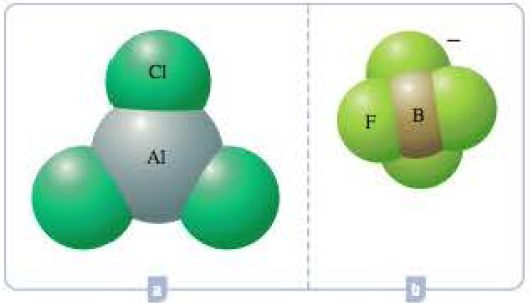
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the major product of
this reaction. Ignore
inorganic
byproducts.
Incorrect, 1 attempt
remaining
1. LiAlH4
2. H3O+
Q
OH
☑
Select to Draw
How should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve.
Software: Excel Spreadsheets
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ug
Provide the proper IUPAC name only for the following
compound. Dashes, commas, and spaces must be used
correctly, but do not use italics in Canvas.
Chapter 10 Solutions
Bundle: General Chemistry, Loose-Leaf Version, 11th + LabSkills PreLabs v2 for Organic Chemistry (powered by OWLv2), 4 terms (24 months) Printed ... for Ebbing/Gammon's General Chemistry, 11th
Ch. 10.1 - An atom in a molecule is surrounded by four pairs...Ch. 10.1 - Use the VSEPR method to predict the geometry of...Ch. 10.1 - Prob. 10.2ECh. 10.2 - Bromine trifluoride, BrF3, has a nonzero dipole...Ch. 10.2 - Which of the following would be expected to have a...Ch. 10.2 - Two molecules, each with the general formula AX3,...Ch. 10.3 - Using hybrid orbitals, describe the bonding in NH3...Ch. 10.4 - Describe the bonding on the carbon atom in carbon...Ch. 10.4 - Dinitrogen difluoride (see Example 10.5) exists as...Ch. 10.4 - Prob. 10.3CC
Ch. 10.6 - The C2 molecule exists in the vapor phase over...Ch. 10.6 - Give the orbital diagram and electron...Ch. 10 - Describe the main features of the VSEPR model.Ch. 10 - According to the VSEPR model, what are the...Ch. 10 - Why is a lone pair expected to occupy an...Ch. 10 - Prob. 10.4QPCh. 10 - Explain why nitrogen trifluoride has a small...Ch. 10 - Prob. 10.6QPCh. 10 - What is the angle between two sp3 hybrid orbitals?Ch. 10 - Prob. 10.8QPCh. 10 - Prob. 10.9QPCh. 10 - How does the valence bond description of a...Ch. 10 - Prob. 10.11QPCh. 10 - What factors determine the strength of interaction...Ch. 10 - Prob. 10.13QPCh. 10 - Prob. 10.14QPCh. 10 - Prob. 10.15QPCh. 10 - Describe the bonding in O3, using molecular...Ch. 10 - Prob. 10.17QPCh. 10 - Which of the following molecular geometries does...Ch. 10 - Which of the following would be a polar molecule?...Ch. 10 - Prob. 10.20QPCh. 10 - Best Lewis Formula and Molecular Geometry A...Ch. 10 - Prob. 10.22QPCh. 10 - Prob. 10.23QPCh. 10 - Which of the following molecular models correctly...Ch. 10 - Prob. 10.25QPCh. 10 - Prob. 10.26QPCh. 10 - Indicate what hybrid orbital depicted below is...Ch. 10 - An atom in a molecule has two bonds to other atoms...Ch. 10 - Two compounds have the same molecular formula,...Ch. 10 - A neutral molecule is identified as a...Ch. 10 - Acetic acid, the sour constituent of vinegar, has...Ch. 10 - What are the bond angles predicted by the VSEPR...Ch. 10 - Predict the shape or geometry of the following...Ch. 10 - Use the electron-pair repulsion model to predict...Ch. 10 - Predict the geometry of the following ions, using...Ch. 10 - Use the VSEPR model to predict the geometry of the...Ch. 10 - For each of the following molecules, state the...Ch. 10 - For each of the following molecules, state the...Ch. 10 - Prob. 10.39QPCh. 10 - From the electron-pair repulsion model, predict...Ch. 10 - Predict the geometries of the following ions,...Ch. 10 - Name the geometries expected for the following...Ch. 10 - a The molecule AsF3 has a dipole moment of 2.59 D....Ch. 10 - a The molecule BrF3 has a dipole moment of 1.19 D....Ch. 10 - Which of the following molecules would be expected...Ch. 10 - Which of the following molecules would be expected...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - a Mercury(II) chloride dissolves in water to give...Ch. 10 - a Nitrogen trifluoride, NF3, is a relatively...Ch. 10 - a Carbonyl fluoride, COF2, is an extremely...Ch. 10 - a The molecule HNNH exists as a transient species...Ch. 10 - The hyponitrite ion, ONNO, exists in solid...Ch. 10 - Fumaric acid, C4H4O4, occurs in the metabolism of...Ch. 10 - Describe the electronic structure of each of the...Ch. 10 - Use molecular orbital theory to describe the...Ch. 10 - Prob. 10.59QPCh. 10 - Write the molecular orbital configuration of the...Ch. 10 - Predict the molecular geometry of the following: a...Ch. 10 - Prob. 10.62QPCh. 10 - Which of the following molecules or ions are...Ch. 10 - Which of the following molecules or ions are...Ch. 10 - Describe the hybrid orbitals used by each carbon...Ch. 10 - Prob. 10.66QPCh. 10 - Explain how the dipole moment could be used to...Ch. 10 - Two compounds have the formula Pt(NH3)2Cl2....Ch. 10 - Explain in terms of bonding theory why all four...Ch. 10 - Explain in terms of bonding theory why all atoms...Ch. 10 - What is the molecular orbital configuration of...Ch. 10 - Prob. 10.72QPCh. 10 - Calcium carbide, CaC2, consists of Ca2+ and C22...Ch. 10 - Sodium peroxide, Na2O2, consists of Na+ and O22...Ch. 10 - The oxygen oxygen bond in O2 is 112 pm and in O2...Ch. 10 - The nitrogennitrogen bond distance in N2 is 109...Ch. 10 - Using molecular orbital theory, determine the...Ch. 10 - The ionization energy of O2 is smaller than the...Ch. 10 - Prob. 10.79QPCh. 10 - Prob. 10.80QPCh. 10 - Prob. 10.81QPCh. 10 - Prob. 10.82QPCh. 10 - What is the biological importance of stratospheric...Ch. 10 - Prob. 10.84QPCh. 10 - Prob. 10.85QPCh. 10 - The bond length in C2 is 131 pm. Compare this with...Ch. 10 - Calcium carbide, CaC2, has an ionic structure with...Ch. 10 - Write Lewis formulas for the BF molecule (two with...Ch. 10 - Boron trifluoride, BF3, reacts with ammonia, NH3,...Ch. 10 - Prob. 10.90QPCh. 10 - Allene (1,2-propadicne), a gas, has the following...Ch. 10 - Prob. 10.92QPCh. 10 - The triiodide ion, I3, and the azide ion, N3, have...Ch. 10 - Hydrogen azide (also known as hydrazoic acid),...Ch. 10 - Prob. 10.95QPCh. 10 - A molecule XF6 (having no lone pairs) has a dipole...Ch. 10 - Describe the molecular orbital configurations of...Ch. 10 - Prob. 10.98QPCh. 10 - Three different compounds have the same molecular...Ch. 10 - Prob. 10.100QPCh. 10 - Prob. 10.101QPCh. 10 - Solid sulfur normally consists of crystals of S8...Ch. 10 - Prob. 10.103QPCh. 10 - Consider the bonding in nitrate ion, NO3. First...Ch. 10 - A molecular compound is composed of 52.5% Xe,...Ch. 10 - A molecular compound is composed of 58.8% Xe,...Ch. 10 - A compound of chlorine and fluorine. ClFn, reacts...Ch. 10 - Excess fluorine, F2(g), reacts at 150C with...Ch. 10 - Prob. 10.109QPCh. 10 - One resonance formula of benzene, C6H6, is What is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forwardFor reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forward
- What is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forwardProvide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forward
- Question 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forwardPlease assign all the carbons for C-NMR and hydrogen for H-NMR. Please if I can get that less than hourarrow_forwardAssign these peaks spectrum ( H-NMR and C-NMR)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY