
Concept explainers
(a)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into
Answer to Problem 10.31P
The
Explanation of Solution
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
The compound
The carboxylic acid,
The alcohol from which the compound
(b)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
Answer to Problem 10.31P
The
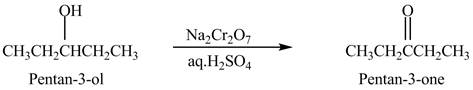
Explanation of Solution
Primary and secondary alcohols are oxidized into aldehydes and ketones using
The secondary alcohols are converted into ketones on oxidation.
The compound
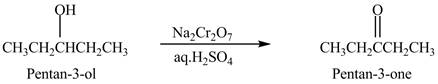
Figure 1
The ketone
The alcohol from which the compound
(c)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
Answer to Problem 10.31P
The
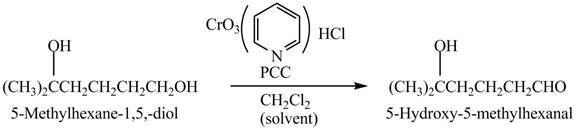
Explanation of Solution
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
The primary alcohols are converted into aldehydes under anhydrous conditions.
The compound

Figure 2
The aldehyde,
The alcohol from which the compound
(d)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
Answer to Problem 10.31P
The
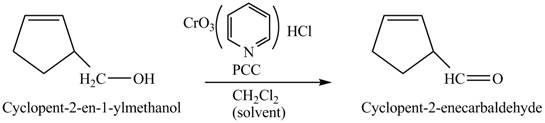
Explanation of Solution
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
The primary alcohols are oxidized into aldehydes under anhydrous conditions.
The compound
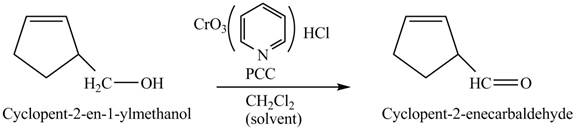
Figure 3
The compound
The alcohol from which the compound
Want to see more full solutions like this?
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forwardPLEASE HELP NOW! URGENT!arrow_forward
- a. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forwardi need help identifying the four carbon oxygen bonds in the following:arrow_forward
- Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forwardHow to solve this!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





