
EBK GENERAL CHEMISTRY
11th Edition
ISBN: 8220103631259
Author: Bissonnette
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 1, Problem 78IAE
Interpretation Introduction
Interpretation:
The density of methanol grams/milliliter is to be determined.
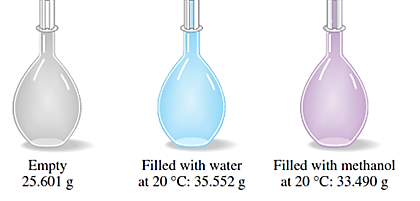
Concept introduction:
- Density is defined as the mass of the substance divided by the volume occupied by the substance.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Steps and explanation
Steps and explanations please.
Steps on how to solve. Thank you!
Chapter 1 Solutions
EBK GENERAL CHEMISTRY
Ch. 1 - What are the principal reasons that one theory...Ch. 1 - Prob. 2ECh. 1 - A common belief among scientists is that there...Ch. 1 - Describe several ways in which a scientific law...Ch. 1 - Describe the necessary characteristics of an...Ch. 1 - Describe the necessary characteristics of a...Ch. 1 - State whether the following properties of matter...Ch. 1 - State whether the following properties are...Ch. 1 - Indicate whether each sample of matter listed is 8...Ch. 1 - Indicate whether each sample of matter listed is...
Ch. 1 - Suggest physical changes by which the following...Ch. 1 - What type of changephysical or chemicalis...Ch. 1 - Express each number in exponential notation....Ch. 1 - Express each number common decimal form. a....Ch. 1 - Express each value in exponential form. Where...Ch. 1 - Express each value in exponential form. Where...Ch. 1 - Indicate whether each of the following is an exact...Ch. 1 - Indicate whether each of the following is an exact...Ch. 1 - Prob. 19ECh. 1 - How many significant figures are shown in each of...Ch. 1 - Perform the following calculations; express each...Ch. 1 - Perform the following calculations; express each...Ch. 1 - Perform the following calculations and retain the...Ch. 1 - Express the result of each of the following...Ch. 1 - An American press release describing the 1986...Ch. 1 - Prob. 26ECh. 1 - Perform the following conversions. a. 0.127L=mL b....Ch. 1 - Prob. 28ECh. 1 - Perform the following from non-SI to SI units....Ch. 1 - Prob. 30ECh. 1 - Which is the greater mass, 3245 (g or 0.00515 mg?...Ch. 1 - Which is the greater mass, 3257 mg or 0.000475 kg?...Ch. 1 - The non-SI unit, hand (used by equestrians), is 4...Ch. 1 - The und furlong is used in horse racing. The unis...Ch. 1 - A sprinter runs the 100 yd dash in 9.3 s. At this...Ch. 1 - A non-SI unit of mass used in Pharmaceutical work...Ch. 1 - Prob. 37ECh. 1 - In an engineering reference book, you find that...Ch. 1 - Prob. 39ECh. 1 - The volumeofaredbloodcell isabout 90.010-12cm3 ....Ch. 1 - We want tomark offathermometer in it Celsius and...Ch. 1 - The highestandlowesttemperatures on record for San...Ch. 1 - The absolute zero of temperature is -273.15C....Ch. 1 - Prob. 44ECh. 1 - Prob. 45ECh. 1 - Prob. 46ECh. 1 - A 2.18 L sample of butyric acid, a substance...Ch. 1 - A 15.2 L sample of chloroform at 20 C has a mass...Ch. 1 - To determine the density of acetone, a 55.0 gal...Ch. 1 - To determine the volume of an irregularly shaped...Ch. 1 - A solution consisting of 8.50% acetone and 91.5%...Ch. 1 - Prob. 52ECh. 1 - A fertilizer contains 21% nitrogen by mass. What...Ch. 1 - A sample is found to have a density of 1.006 g/mL,...Ch. 1 - Prob. 55ECh. 1 - Calculate the mass of a cylinder of stainless...Ch. 1 - The densities are given at 20 C: water, 0.998g/cm3...Ch. 1 - To determine the approximate mass of a small...Ch. 1 - The density of aluminum is 2.70 g/ cm3. A square...Ch. 1 - The angle iron pictured here is made of steel with...Ch. 1 - In normal blood, there are about 5.4109 red blood...Ch. 1 - A technique once used by geologists to measure the...Ch. 1 - In a class of 76 students, the results of...Ch. 1 - A class of 84 students had a final grade...Ch. 1 - Prob. 65ECh. 1 - A solution containing 12.0% sodium hydroxide by...Ch. 1 - According to the rules on significant figures, the...Ch. 1 - Prob. 68IAECh. 1 - A solution used to chlorinate a home swimming pool...Ch. 1 - A standard 1.000 kg mass is to be tut from a bar...Ch. 1 - Prob. 71IAECh. 1 - Prob. 72IAECh. 1 - Magnesium occurs in seawater to the extent of 1.4...Ch. 1 - A typical rate of deposit of dust ("dustfall")...Ch. 1 - In the United States, the volume of irrigation...Ch. 1 - A Fahrenheit and a Celsius thermometer are...Ch. 1 - The accompanying illustration shows e 100.0 mL...Ch. 1 - Prob. 78IAECh. 1 - Prob. 79IAECh. 1 - A pycnometer (see Exercise 78) weighs 25.60 g...Ch. 1 - The Greater Vancouver Regional District (GVRO)...Ch. 1 - A Boeing 767 due to fly from Montreal to Edmonton...Ch. 1 - The following equation can be used to relate the...Ch. 1 - Prob. 84IAECh. 1 - A tabulation of datalists the following equation...Ch. 1 - The total volume of ice in the Antarctic is about...Ch. 1 - An empty 3.00 L bottle weighs 1.70 kg. Fled with...Ch. 1 - The filament in an incandescent light bulb is made...Ch. 1 - Blood alcohol content (BAC) is sometimes reported...Ch. 1 - In an attempt to determine any possible...Ch. 1 - Prob. 91FPCh. 1 - Prob. 92FPCh. 1 - The canoe gliding gracefully along the water in...Ch. 1 - The accompanying sketches suggest four...Ch. 1 - As mentioned on page 13, the MCO was lost because...Ch. 1 - In your own words, define or explain the following...Ch. 1 - Prob. 97SAECh. 1 - Explain the important distinctions between each...Ch. 1 - A procedure designed to test the truth or the...Ch. 1 - The fact that the volume of a fixed amount of gas...Ch. 1 - If a sample of matter cannotbe separated by...Ch. 1 - A good example of a homogeneous mixture is a. a...Ch. 1 - Compared withits mass on Earth, the mass of the...Ch. 1 - Which answer has the correct number of significant...Ch. 1 - Which two of the following masses are expressed to...Ch. 1 - Prob. 106SAECh. 1 - Prob. 107SAECh. 1 - Prob. 108SAECh. 1 - The density of water is 0.9982 g/cm2 at 20C....Ch. 1 - Two students each made four measurements of the...Ch. 1 - Prob. 111SAECh. 1 - List the blowing the order of increasing...Ch. 1 - Without doing detailed calculations, explain which...Ch. 1 - Prob. 114SAECh. 1 - Water, acompound, is a substance. Is there any...Ch. 1 - In the production of ammonia, the...Ch. 1 - Appendix E descries a useful study aid known as...
Knowledge Booster
Similar questions
- 3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward1. Which one(s) of these can be oxidized with CrO3 ? (could be more than one) a) triphenylmethanol b) 2-pentanol c) Ethyl alcohol d) CH3 2. Write in all the product(s) of this reaction. Label them as "major" or "minor". 2-methyl-2-hexanol H2SO4, heatarrow_forward3) Determine if the pairs are constitutional isomers, enantiomers, diastereomers, or mesocompounds. (4 points)arrow_forward
- In the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
